| Product Name | NpFR1 |
| Description |
Reversible fluorescence intensity-based redox sensor |
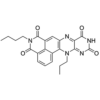
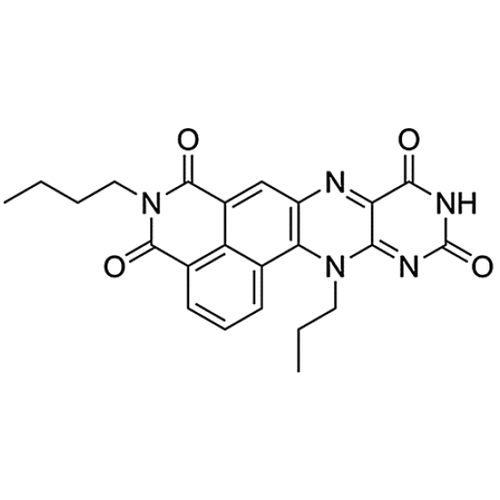
| Molecular Formula | C23H21N5O4 |
| Molecular Weight | 431.45 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Blue Ice or 4ºC |
| Product Type | Redox Probe |
| Solubility | Soluble in DMSO |
| Source | Synthetic |
| Appearance | Red Solid |
| SMILES | C1(N(C(C2=CC5=C(C3=CC=CC1=C23)N(C4=NC(NC(C4=N5)=O)=O)CCC)=O)CCCC)=O |
| InChI | InChI=1S/C23H21N5O4/c1-3-5-10-28-21(30)13-8-6-7-12-16(13)14(22(28)31)11-15-18(12)27(9-4-2)19-17(24-15)20(29)26-23(3 |
| InChIKey | C5(C2=C1C(=CC4=C(C1=CC=C2)N(C3=NC(NC(C3=N4)=O)=O)CCC)C(N5CCCC)=O)=O |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | NpFR1 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-181) |
Biological Description
| Research Areas | Cancer, Oxidative Stress |
| Scientific Background | NpFR1, or napthalimide-flavin redox sensor 1, is a novel flavin molecule that exhibits a greater than 100-fold increase in fluorescence upon oxidation, and is easily reversible. In the oxidative form, is shows a maximum absorption at 395 and 463nm, max admission at 545nm. Treatment with a mild reducing agent (including sodium thiosulfate, sodium cyanoborohydride, DTT and glutathione) gave the reduced form which exhibited 110-fold lower absorbance, and 125-fold lower emission. Re-oxidation to its original fluorescence can be achieved by air or by hydrogen peroxide. |
| References | 1. Yeow J., Kaur A.K., Anscomb M.D., and New E.J. (2014) Chem. Commun. 50: 8181-8184. |
Product Images
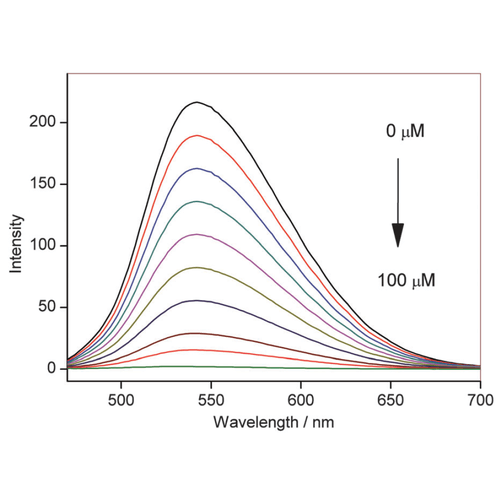
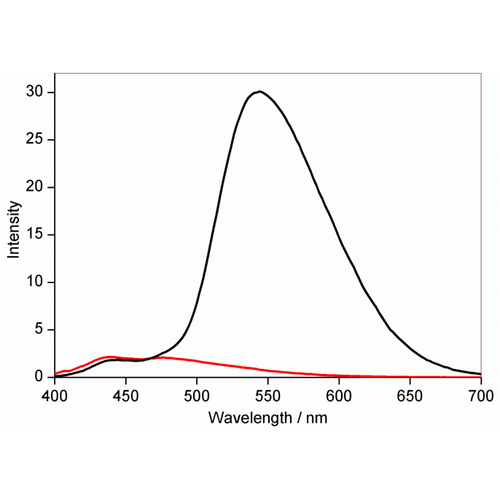
Fluorescence emission of NpFR1 (SIH-181, 5 mM, lex = 405 nm) with the incremental addition of sodium dithionite. All spectra were acquired in HEPES buffer (100 mM, pH 7.4). This image is from Chem. Commun., 2014, 50, 8181, and licensed under a Creative Commons Attribution 3.0 Unported Licence (http://creativecommons.org/licenses/by/3.0/).
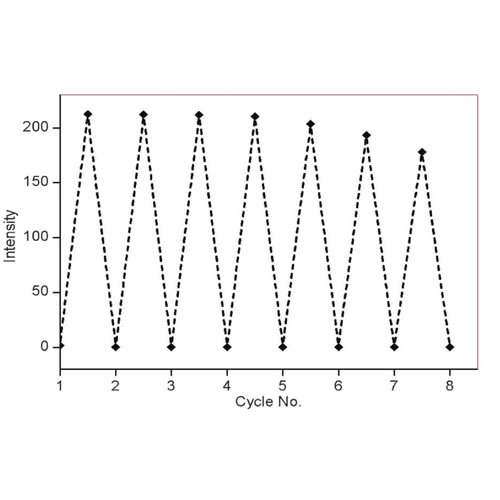
Fluorescence response of NpFR1 (SIH-181) to cycles of oxidation and reduction. Reduction was achieved with sodium dithionite (100mM) followed by re-oxidation with 250mM H2O2. Spectra were recorded 5 min after the addition of reducing and oxidising agents. All spectra were acquired in HEPES buffer (100 mM, pH 7.4). This image is from Chem. Commun., 2014, 50, 8181, and licensed under a Creative Commons Attribution 3.0 Unported Licence (http://creativecommons.org/licenses/by/3.0/).
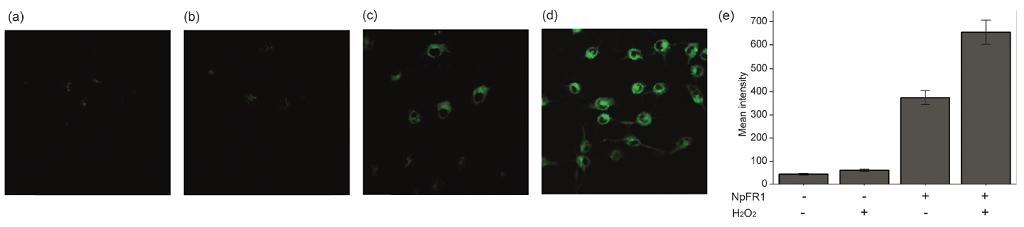
Imaging of NpFR1 (SIH-181) in 3T3-L1 preadipocytes treated with (a) vehicle control (b) H2O2 (100 mM, 2 min), (c) NpFR1 (50 mM, 2 h) and (d) NpFR1 (50 mM, 2 h) followed by H2O2 (100 mM, 2 min). Scale bar represents 50 mm, lex = 405 nm. (e) Integrated emission from 510 nm to 610 nm. Values are the mean ratio generated from the intensity from five fields of cells. Error bars represent standard error measurement (s.e.m.). The layout of this image was modified for optimal display from the original Chem. Commun., 2014, 50, 8181, and licensed under a Creative Commons Attribution 3.0 Unported Licence (http://creativecommons.org/licenses/by/3.0/).




Reviews
There are no reviews yet.