Active Tau Pre-formed Fibrils for Alzheimer’s Disease Research
StressMarq is proud to be a world leader in the development and supply of fibrilized & oligomeric proteins for researchers studying Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. We are the first to offer active tau pre-formed fibrils (PFFs) for neuroscience research and continue to expand our portfolio of tau products.
Tau proteins are primarily located in axons and stabilize microtubules in neurons. In certain neurodegenerative diseases, known as tauopathies, tau becomes detached from microtubules and forms insoluble aggregates in the brain, known as neurofibrillary tangles (NFTs). Because tau can no longer stabilize microtubules in this form, and tau oligomers and tangles can be neurotoxic, neurons cease to function and die. StressMarq now offers two monomers and two PFFs that allow scientists to induce and study this effect in vitro. This gives them the potential to investigate the mechanisms of tau aggregation, the effect of various tau species on neurons, and ability of certain drugs to inhibit tau aggregation.
The process of tau aggregation can be seeded by active tau PFFs, which recruit monomers to form larger tau fibrils. This has been demonstrated in thioflavin T assays where an increase in fluorescence, indicative of tau fibrillization, is seen when active tau PFFs are combined with active tau monomers. The seeding process is similar to that of alpha synuclein, which can also be used to study neurodegenerative diseases.
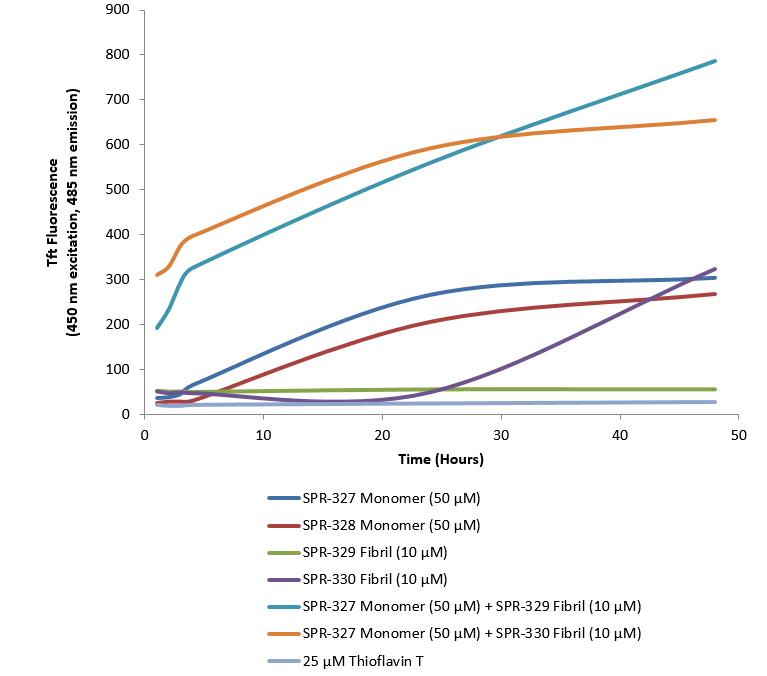
Thioflavin T is a fluorescent dye that binds to beta sheet-rich structures, such as those in tau fibrils. Upon binding, the emission spectrum of the dye experiences a red-shift and increased fluorescence intensity. Thioflavin T emission curves show increased fluorescence (correlated to tau aggregation) over time in tau preformed fibrils (catalog# SPR-330). Tau preformed fibrils seed the formation of new tau fibrils when combined with tau monomers (catalog# SPR-327).
Monomers and fibrils are available both in the full-length isoform of the tau protein (2N4R or Tau-441) or a truncated form (K18). Tau-441 has a molecular weight of approximately 46 kDa, whereas K18 tau has a molecular weight of approximately 15 kDa. Both full-length and truncated PFFs induce tau aggregation; full-length PFFs may be more effective in seeding fibrillization, but a combination of both can be particularly toxic to neurons.1 All proteins are untagged and expressed in E. coli.
The full-length tau monomers and fibrils have P301S mutations and the truncated monomers and fibrils have P301L mutations. Both mutations occur in exon 10 and are associated with frontotemporal dementia. The P301S mutation reduces tau’s ability to assemble microtubules, and the P301L mutation promotes beta-sheet formation and the formation of PHFs.2 Both P301S and P301L mutant transgenic mouse models are used in tau research.
Learn more about tau aggregation and types of tau fibrils for Alzheimer’s Disease research
References
- Ozcelic, S. et al. Mol Psychiatry. 2016, 21(12): 1790–1798.
- alzforum.org/mutations/mutation-position-table/mapt-p301-mutations


Are your tau products endotoxin-free?
Hello Leon,
Our tau products have had endotoxin removed via ion-exchange. Based on testing so far, endotoxin levels are expected to be between 10 and 20 EU/mL. Please feel free to comment or email info@stressmarq.com with any other questions.
Best regards,
Patricia