Alpha Synuclein Monomers: Why Target Them?
Alpha synuclein is the product of the SNCA gene and is expressed as a 14kDa monomer. This comprises an N-terminal domain, a nonamyloid-𝛽 component of plaques (NAC) domain, and a C-terminal domain, of which the NAC domain distinguishes alpha synuclein from other members of the synuclein family1. Most notably, a short sequence of amino acids (residues 71-82) within the NAC domain provides alpha synuclein with the capacity to self-polymerize into fibrils2. Within neurons, fibrils promote the recruitment of soluble endogenous alpha synuclein to form structures known as Lewy bodies and Lewy neurites – the classic pathological hallmarks of Parkinson’s disease (PD)3. By targeting monomeric alpha synuclein for therapeutic intervention, researchers aim to prevent the irreversible neurodegeneration underlying PD and other synucleinopathies.
Why target alpha synuclein monomers?
Alpha synuclein has long been known for its association with Parkinson’s disease. In 1997, a missense mutation (A53T) in the SNCA gene was shown to contribute to familial PD, and many other PD-associated alpha synuclein mutations have since been identified4,5. These are suggested to promote PD pathogenesis through mechanisms that include destabilizing the encoded monomers, or causing their over-expression, such that the likelihood of aggregate formation is increased5,6. Another important link between alpha synuclein and Parkinson’s disease comes from the discovery that deleting portions of the NAC domain impairs the ability of alpha synuclein to form fibrils7. Findings like these are of critical importance in developing effective therapeutic strategies. To date, approaches targeting alpha synuclein monomers have been designed to reduce their expression, prevent them from forming aggregates, or promote their degradation. As just one example, using an engineered protein (AS69) known to bind monomeric alpha synuclein with high affinity, researchers have demonstrated reduced aggregate formation in cultured HEK293T cells co-expressing AS698.
How can alpha synuclein monomers be used in aggregation assays?
Because native alpha synuclein is an intrinsically disordered protein, designing small molecule drugs to interact with it is extremely challenging. This has led some researchers to use surface plasmon resonance (SPR) for investigating the interaction between alpha synuclein monomers (that are free in solution) and small molecule drugs tethered on microchips, with interesting ‘hits’ subsequently being progressed for further evaluation9. Downstream analysis often involves performing aggregation assays, where pre-formed alpha synuclein fibrils are seeded into a solution of monomeric alpha synuclein and the small molecule drug of interest. Where the small molecule drug inhibits fibril formation, the fluorescent signal from Thioflavin T (a dye that binds beta sheet-rich structures such as those found in alpha synuclein fibrils) is reduced compared to a control. Recently, using aggregation assays to test hits from a high-throughput screen to identify tau-binding compounds, researchers have highlighted dual targeting of tau and alpha synuclein as a promising strategy for treating a broad range of neurodegenerative diseases10.
High quality reagents for studying alpha synuclein aggregation
StressMarq offers a comprehensive selection of reagents for studying alpha synucleinopathies such as Parkinson’s disease. These include our active human recombinant alpha synuclein protein monomers – both Type 1 (SPR-321) and Type 2 (SPR-316) – and the equivalent mouse Type 1 protein (SPR-323). In addition, we have developed an active human recombinant A53T mutant (SPR-325) to further support your research.
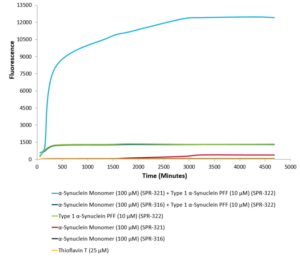
Type 1 alpha synuclein preformed fibrils (SPR-322) seed the formation of new alpha synuclein fibrils from a pool of alpha synuclein monomers (SPR-321). Thioflavin T emission curves show increased fluorescence over time when 10 µM of Type 1 alpha synuclein preformed fibrils (SPR-322) is combined with 100 µM of alpha synuclein monomer (SPR-321), as compared to when 10 µM of Type 1 alpha synuclein preformed fibrils (SPR-322) is combined with 100 µM of alpha Synuclein monomer (SPR-316 ).
REFERENCES
- Alpha-Synuclein in Parkinson’s Disease: From Pathogenetic Dysfunction to Potential Clinical Application, Xu L and Pu J, Parkinsons Dis. 2016;2016: 1720621
- A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly, Giasson BI et al, J Biol Chem. 2001 Jan 26;276(4):2380-6
- Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death, Volpicelli-Daley LA et al, Neuron. 2011 Oct 6;72(1):57-71
- Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease, Polymeropoulos MH et al, Science. 1997 Jun 27;276(5321):2045-7
- New Perspectives on Roles of Alpha-Synuclein in Parkinson’s Disease, Zhang G et al, Front Aging Neurosci. 2018 Nov 22;10:370
- β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease, Mittal S et al, Science. 2017 Sep 1;357(6354):891-898
- Residue Glu83 Plays a Major Role in Negatively Regulating α-Synuclein Amyloid Formation, Waxman EA et al, Biochem Biophys Res Commun. 2010 Jan 15;391(3):1415-20
- An engineered monomer binding-protein for α-synuclein efficiently inhibits the proliferation of amyloid fibrils, Agerschou ED et al, eLife 2019;8:e46112
- Novel Small Molecules Targeting the Intrinsically Disordered Structural Ensemble of α-Synuclein Protect Against Diverse α-Synuclein Mediated Dysfunctions, Tóth G et al, Sci Rep. 2019 Nov 18;9(1):16947
- Dual Targeting of Monomeric Tau and α-Synuclein Aggregation: A New Multitarget Therapeutic Strategy for Neurodegeneration, Gabr MT and Peccati F, ACS Chem Neurosci. 2020 Jul 15;11(14):2051-2057


Leave a Reply