Alpha Synuclein Oligomers and their Role in Synucleinopathies
Alpha synuclein, an abundant synaptic protein, has been inextricably linked to Parkinson’s disease and other synucleinopathies involving Lewy bodies. This 14 kDa protein, expressed by the SNCA gene, is one of the main filamentous components of Lewy bodies; inclusions in the brain found in specific neurodegenerative disorders (1).
Alpha synuclein is a 140 amino acid protein expressed ubiquitously in neurons (2). It has a three-domain structure consisting of an N-terminal domain of seven repeating peptides, a central non-amyloid–beta component (NAC) domain, and an unstructured C-terminus. When present in the cytosol as a monomer, alpha synuclein takes a mainly disordered conformation. On binding to a membrane via the protein’s N-terminal lipid-binding domain, an alpha-helical structure is adopted (3). The normal function of alpha synuclein is not fully elucidated, but suggested roles include the regulation of the SNARE complex, which in the pre-synapse controls neurotransmitter release (1) and association with proteins such as tubulin in the brain (4).
A genetic link to disease was established on the discovery that N-terminal SNCA point mutations, duplications and triplications of the SNCA gene, all lead to early onset alpha-synucleinopathies. A number of these point mutations have been shown to promote the formation of alpha synuclein oligomers, or pre-fibrils (1, 3). These large soluble protein aggregates of alpha synuclein are thought to have neurotoxic properties and be responsible for the deterioration of the brain observed in alpha synucleinopathies.
Alpha synuclein oligomer toxicity
The α-synuclein cascade hypothesis states that the normally disordered alpha synuclein monomers aggregate into soluble oligomers (proto-fibrils). However, if further aggregation continues, they form ordered insoluble fibrils with a predominantly beta sheet structure, such as those found in Lewy bodies. It is the oligomeric intermediate form which is believed to exert the most toxic effects (1). Through the study of Parkinson’s disease progression, it was established that alpha synuclein oligomers are able to seed and spread through the nervous system in a prion-like manner, from affected neurons to unaffected neurons (5).
Research shows oligomers range in size, structure, their ability to seed and their toxicity. This toxicity is due to the accumulation of protein in the synapses disrupting a multitude of processes leading to cell death. The pathways thought to be affected include those of the mitochondria, the endoplasmic reticulum leading to chronic ER stress, cellular homeostasis by pore formation in the cell membrane, autophagic and lysosomal function leading to further oligomer accumulation, and the SNARE complex leading to impaired neurotransmitter release (1, 6).
Recombinant alpha synuclein oligomers in Parkinson’s Disease research
When treated with agents such as Epigallocatechin-3-gallate (EGCG) or dopamine, it is possible to produce stable recombinant a-syn oligomers that do not further aggregate into fibrils. EGCG promotes the formation of non-toxic oligomers, and it has been reported that immunization with EGCG-stabilized recombinant alpha synuclein can improve symptoms in a transgenic mouse model of Parkinson’s disease (6). Whereas dopamine promotes self-aggregation (7), can cross-seed amyloid-beta aggregates (8) and has been shown to induce neurodegeneration in worm and mouse in vivo models (9).
StressMarq offers a range of alpha synuclein oligomers, developed for research into Parkinson’s disease and other alpha-synucleinopathies. Our Alpha Synuclein Oligomers (Epigallocatechin Gallate Stabilized) (catalog# SPR-469) have been shown to not seed alpha synuclein monomer (catalog# SPR-321) in Thioflavin T assays. Our dopamine HCL stabilized oligomers (catalog# SPR-466) have demonstrated toxicity on primary mouse cortical neurons (see figures below). Finally, our new Alpha Synuclein Kinetically Stable Oligomers (catalog# SPR-484) have demonstrated toxicity and are generated without inducers or inhibitors.
For more information and details of additional alpha synuclein products in our range, please visit our website.
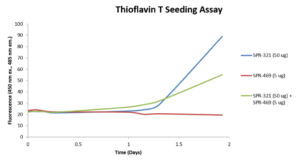
Thioflavin T assay of EGCG-stabilized alpha synuclein oligomers (SPR-469) and alpha synuclein monomers (SPR-321). The oligomers do not seed aggregation of monomers and may have an inhibitory effect.
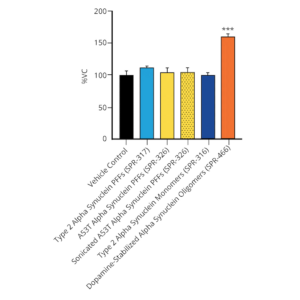
Evaluation of a-syn toxicity on primary mouse cortical neurons. Lactate dehydrogenase (LDH) is a soluble enzyme present in the cytosol that is released upon cell death. Toxicity was assessed with an LDH assay and displayed as % of vehicle control (VC). Data are presented as bar graphs and standard deviation. For statistical analysis One-way ANOVA followed by Bonferroni post-hoc test (vs VC) was used. *** p<0.001. Treatment with dopamine-stabilized oligomers was accompanied by a significant increase of LDH release. Data courtesy of QPS (qpsneuro.com).
REFERENCES
1) Ingelsson, M. Front Neurosci. 2016; 10:408.
2) Jakes, R. et al. FEBS Lett. 1994; 345(1):27‐32.
3) Zhang, G. et al. Front Aging Neurosci. 2018; 10:370.
4) Alim, M.A. et al. J Biol Chem. 2002; 277(3):2112-2117.
5) Rey, N.L. et al. Acta Neuropathol. 2013; 126:555–573.
6) Bengoa-Vergniory, N. et al. Acta Neuropathol. 2017; 134(6):819-838.
7) Pieri, L. et al. Sci Rep. 2016; 6:24526.
8) Planchard, M.S. et al. Protein Sci. 2014; 23(10):1369-79.
9) Mor, D.E. et al. Nat Neurosci. 2017; 20(11):1560-1568.


Leave a Reply