Alpha Synuclein in Parkinson’s Disease Diagnostics
Despite being the second most common neurodegenerative disorder, Parkinson’s disease currently has no cure. One reason for this is that, currently, Parkinson’s disease can only accurately be diagnosed post-mortem. This makes it extremely difficult to stratify patients for recruitment into clinical trials aimed at progressing potential therapeutics1. To address this issue, significant research efforts are being made to identify Parkinson’s disease biomarkers. These would allow Parkinson’s disease to be differentiated from other related disorders and could ultimately be used to direct an appropriate course of treatment. Of the potential biomarker candidates currently under investigation, it is alpha synuclein that has received the most interest. Not only is aggregated alpha synuclein the major component of Lewy bodies and Lewy neurites – the classic neuropathological hallmarks of Parkinson’s disease, found in the brain – but also, mutations in the gene (SNCA) encoding alpha synuclein have been linked to aggregate formation.
How could alpha synuclein be used to diagnose Parkinson’s Disease?
There are several different ways that alpha synuclein might be used to diagnose Parkinson’s disease. The first of these is to measure different forms of the protein in bodily fluids such as serum or plasma, comparing alpha synuclein concentrations to control levels. Indeed, a recent study has shown serum alpha synuclein levels to correlate moderately with motor severity in patients with early Parkinson’s disease, highlighting the utility of a larger, multi-center study2. Another approach is to investigate alpha synuclein levels in peripheral tissues like salivary glands or the olfactory mucosa – tissues that are more amenable to biopsy than the brain – although research has indicated that certain forms of alpha synuclein may be more useful than others as Parkinson’s disease biomarkers using such samples 3,4. It may also be possible to evaluate the seeding activity (the release of misfolded protein seeds and subsequent uptake by anatomically connected neurons) of alpha synuclein isolated from cerebrospinal fluid (CSF), with a 2019 review suggesting this method to have shown promise in pilot studies5. Additionally, building on the success of amyloid‐beta imaging in Alzheimer’s disease patients, work is ongoing to evaluate means of imaging alpha synuclein in the brain6,7,8.
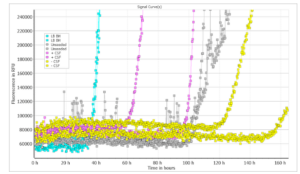
Type 2 monomers (SPR-316) are currently undergoing testing in a Real-Time Quaking-Induced Conversion (RT-QuIC) assay. At 10 μg/well there was discrimination between positive and negative CSF samples, and the unseeded reaction occurred later than either the LB BH or the positive CSF sample. This suggests Type 2 monomers could potentially be used as a substrate for alpha synuclein RT-QuIC. Further testing/optimization is underway. LB BH: 10% Lewy body disease; CSF +: CSF from patient with neuropathologically confirmed alpha-synucleinopathy; CSF -: CSF from patient with no evidence of alpha-synuclein deposition at postmortem. Image source: Alison Green, Graham Fairfoul
What are the potential approaches and how do they work?
To date, various methods have been employed to detect and quantify alpha synuclein. These include enzyme‐linked immunosorbent assay (ELISA), western blot, mass spectrometry and Luminex assays for identifying different forms of the protein in bodily fluids; the seeding studies and imaging methods previously noted; and a novel real‐time quaking‐induced conversion (RT‐QuIC) assay that has shown significant promise for detecting aggregated alpha synuclein in CSF9,10. The latter approach exploits the ability of aggregated alpha synuclein to induce further alpha synuclein aggregation in a cyclic manner and has demonstrated an overall specificity of 100% when compared to control CSF material.
Supporting the study of alpha synuclein in Parkinson’s disease
We offer a comprehensive selection of fibrilized and oligomeric proteins and antibodies to support Parkinson’s disease research. These include our Alpha Synuclein Pre-formed Fibrils that seed the formation of new fibrils from alpha synuclein monomers, and our human alpha synuclein Type 2 monomer (SPR-316) that has shown potential as a substrate for alpha synuclein RT-QuIC.
REFERENCES
- Parkinson’s disease biomarkers based on α‐synuclein, Fayyad M. et al. J Neurochem. 2019 Sep;150(5):626-636.
- Plasma and Serum Alpha-Synuclein as a Biomarker of Diagnosis in Patients With Parkinson’s Disease, Chang C.W. et al. Front Neurol. 2020 Jan 21;10:1388.
- Phosphorylated α-synuclein immunoreactivity in nerve fibers from minor salivary glands in Parkinson’s disease, Carletti R. et al. Parkinsonism Relat Disord. 2017 May;38:99-101.
- Lewy body pathology involves the olfactory cells in Parkinson’s disease and related disorders, Saito Y. et al. Mov Disord. 2016 Jan;31(1):135-8.
- Diagnostic biomarkers for Parkinson’s disease: focus on α-synuclein in cerebrospinal fluid, Kalia L.V. Parkinsonism Relat Disord. 2019 Feb;59:21-25.
- The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease-Review of Literature and Interesting Images, Suppiah A. et al. Diagnostics (Basel). 2019 Jun 25;9(2):65.
- Advances in the development of imaging probes and aggregation inhibitors for alpha-synuclein, Xu M.M. et al. Acta Pharmacol Sin. 2020 Apr;41(4):483-498.
- α-synuclein imaging: a critical need for Parkinson’s disease research, Eberling J.L. et al. J Parkinsons Dis. 2013;3(4):565-7.
- Cerebrospinal fluid biomarker for Parkinson’s disease: An overview, Maass F. et al. Mol Cell Neurosci. 2019 Jun;97:60-66.
- Alpha‐synuclein RT‐QuIC in the CSF of patients with alpha‐synucleinopathies, Fairfoul G. et al. Ann Clin Transl Neurol. 2016 Aug 28; 3(10):812-818.


Leave a Reply