Alpha Synuclein in Parkinson’s Disease
Alpha synuclein is encoded by the SNCA gene, which gives rise to a 14kDa protein with an N-terminal domain (aa 1–65), a nonamyloid-? component of plaques (NAC) domain (aa 66–95), and a C-terminal domain (aa 96–140)1. Of these, the NAC domain makes alpha synuclein unique to the other two members of the synuclein family (beta synuclein and gamma synuclein), providing it with the capacity to form fibrils through self-polymerization,2. Since aggregates of fibrillar alpha-synuclein define several neurodegenerative conditions, including Parkinson’s disease, preventing these from developing represents a promising therapeutic approach.
How does alpha synuclein aggregation lead to neurodegeneration in Parkinson’s Disease?
Parkinson’s disease is characterized by progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta of the brain, resulting in the loss of fine motor control and producing symptoms such as resting tremor, postural instability, and muscular rigidity3. A role for alpha synuclein in this process was first described in 1997, following the discovery of a missense mutation (A53T) that could be linked to familial Parkinson’s disease,4. Since then, a growing number of N-terminal missense mutations in alpha synuclein have been associated with Parkinson’s disease, including A30P, E46K, H50Q, G51D, A53E, A18T and pA29S5.
One way that these mutations have been suggested to contribute to Parkinson’s disease is by destabilizing alpha synuclein and increasing its propensity to form aggregates5. However, often by using various mutant forms of alpha synuclein, many other hypotheses have been put forward to explain the protein’s pathogenic role. Firstly, alpha synuclein may alter cytoskeletal dynamics, for example binding actin to alter cellular trafficking and impact synaptic function6. Alpha synuclein has also been proposed to impact protein degradation, a concept that makes sense since conditions such as Parkinson’s disease are often associated with impaired protein clearance7.
Another way that alpha synuclein is thought to contribute to Parkinson’s disease pathogenesis is by inducing the production of reactive oxygen species (ROS), leading to the loss of neuronal cells due to oxidative stress. Supporting this notion, curcumin – a powerful antioxidant – has been shown to protect against A53T-induced cell death8. In addition, alpha synuclein has been shown to target the endoplasmic reticulum and Golgi apparatus; to promote neurotoxicity by inhibiting histone acetylation; and to demonstrate prion-like behavior that could explain the propagation of Parkinson’s disease pathology9,10,11.
Supporting the study of alpha synuclein in Parkinson’s disease
StressMarq’s product portfolio contains a comprehensive selection of high-quality reagents to support Parkinson’s disease research. These include active human (SPR-322) and mouse (SPR-324) recombinant alpha synuclein pre-formed fibrils, active human recombinant A53T mutant alpha synuclein pre-formed fibrils (SPR-326), and dopamine HCL stabilized human recombinant alpha synuclein oligomers (SPR-466).
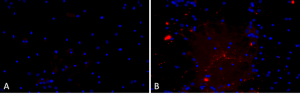
Primary rat hippocampal neurons show Lewy body inclusion formation when treated with A53T mutant Alpha Synuclein Protein Preformed Fibrils (SPR-326) (B) but not when treated with a media control (A). Tissue: Primary hippocampal neurons. Species: Sprague-Dawley rat. Primary Antibody: Rabbit anti-pSer129 Antibody. Fibrils were diluted to 1 ug/uL in neuronal media consisting of B27, Glutamax, penicillin/strip, and neurobasalA and sonicated for 1 hour in a water bath. The sonicated stock was then used to achieve the final concentration of 1 ug/mL in the wells.
REFERENCES
- Alpha-Synuclein in Parkinson’s Disease: From Pathogenetic Dysfunction to Potential Clinical Application, Xu L and Pu J, Parkinsons Dis. 2016;2016
- A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly, Giasson BI et al, J Biol Chem. 2001 Jan 26;276(4):2380-6
- Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease, Zeng XS et al, Front Aging Neurosci. 2018 Apr 17;10:109
- Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease, Polymeropoulos MH et al, Science. 1997 Jun 27;276(5321):2045-7
- New Perspectives on Roles of Alpha-Synuclein in Parkinson’s Disease, Zhang G et al, Front Aging Neurosci. 2018 Nov 22;10:370
- {alpha}-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics, Sousa VL et al, Mol Biol Cell. 2009 Aug;20(16):3725-39
- Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing, Boland B et al, Nat Rev Drug Discov. 2018 Sep;17(9):660-688
- Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism, Liu Z et al, Pharmacol Res. 2011 May;63(5):439-44
- Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion, Gosavi N et al, J Biol Chem. 2002 Dec 13;277(50):48984-92
- Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity, Kontopoulos E et al, Hum Mol Genet. 2006 Oct 15;15(20):3012-23
- Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells, Luk KC et al, Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20051-6


Leave a Reply