Amyloid Beta
StressMarq Biosciences has developed an extensive range of fibrillar, oligomeric and monomeric protein preparations for use in neurodegenerative disease research including alpha synuclein, beta synuclein, gamma synuclein, tau, amyloid beta, SOD1 and TTR. Our goal is to be the world leader in the development and supply of active, pathology-inducing protein aggregates to assist scientists with disease model development and accelerate neurodegenerative disease drug discovery.
In the brain, amyloid beta peptide (Aβ) is generated by protease cleavage of amyloid precursor protein (APP), which aggregates into oligomers, protofibrils, fibrils and ultimately plaques in neurodegenerative diseases. The accumulation of Aβ plaques in the brain is considered a hallmark of Alzheimer’s disease (AD), and most of the drugs tested for AD in the past 20 years have targeted amyloid beta accumulation (3). Soluble Aβ oligomers isolated from the brains of AD patients or those generated in vitro potently impaired synapse structure and function (4). Aβ oligomers generated in vitro were toxic to PC12 cells (2) and SH-SY5Y cells (5). Aβ was demonstrated to interact with tauopathies to affect neurodegeneration in AD patients (6) and accumulations of Aβ were shown to be associated with lower survival rates in Parkinson’s disease patients with dementia (7).
Amyloid Beta Pre-formed Fibrils (PFFs), Oligomers, & Monomers
StressMarq is pleased to offer a variety of amyloid beta protein constructs for neurodegenerative disease research.
Product List | Amyloid Beta
| Human Amyloid Beta Pyroglutamate 3-42 Pre-formed Fibrils (PFFs), catalog# SPR-492 New! |
| Human Amyloid Beta 1-42 Pre-formed Fibrils (PFFs), catalog# SPR-487 |
| Human Amyloid Beta 1-42 Oligomers, catalog# SPR-488 |
| Human Amyloid Beta 1-42 Peptide (HFIP, monomeric), catalog# SPR-485 |
Selected Scientific & Product Information
Structure
StressMarq’s amyloid beta peptide 1-42 (Aβ42) is produced synthetically and treated with 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) prior to drying which breaks down pre-formed fibrils and monomerizes the peptide, as previously published (1,2). Our Amyloid Beta 1-42 (Aβ42) Oligomers and Amyloid Beta 1-42 (Aβ42) Pre-formed Fibrils (PFFs) are generated from this Amyloid Beta Peptide 1-42 (Aβ42) using a previously published method (1,2).
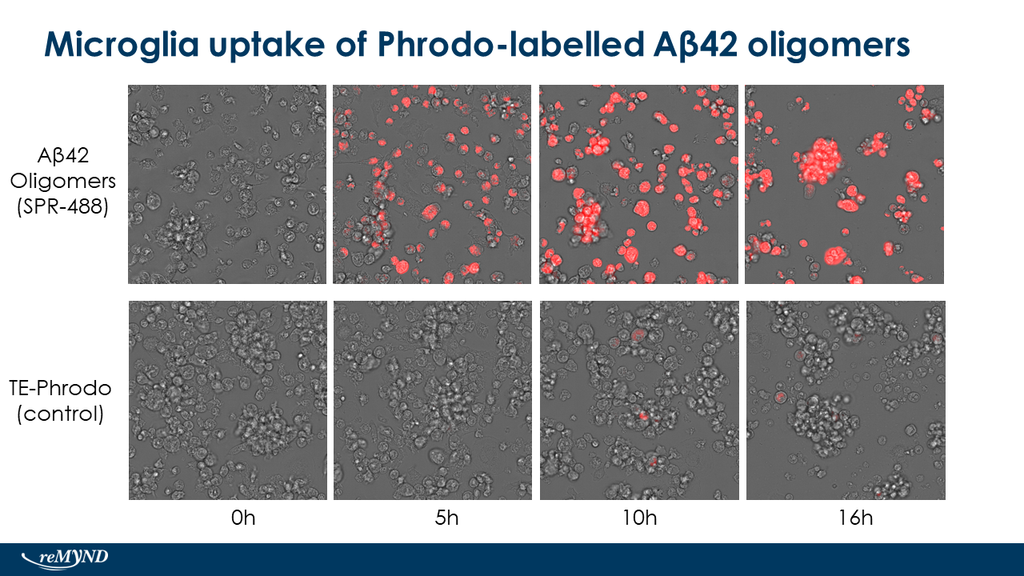
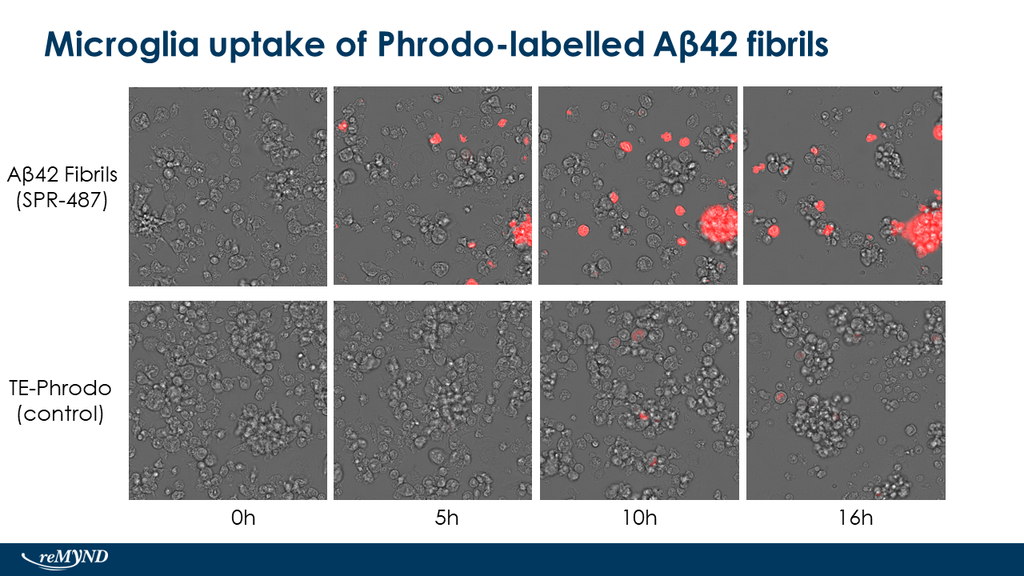
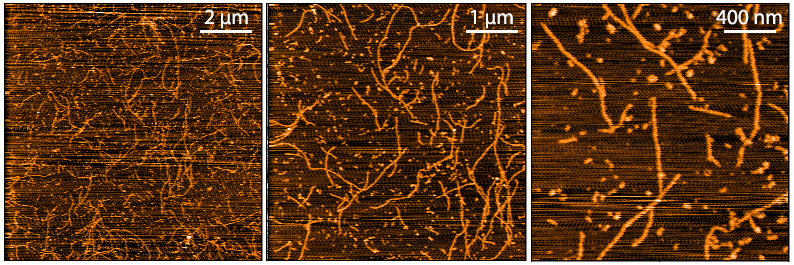
Upon resuspension in DMSO/dH2O, our Aβ42 presents as a monomeric peptide without fibrils when observed under TEM, AFM and on a Western Blot with an anti-amyloid beta antibody. StressMarq’s Aβ42 PFFs present as long strands when observed under TEM and AFM, and have a unique high molecular weight signal on a Western Blot with an anti-amyloid beta antibody. Our Aβ42 oligomers present as globular oligomers when observed under TEM and AFM, and have a unique dimer/trimer and oligomer signal on a Western Blot with an anti-amyloid beta antibody.
TEM of amyloid beta 1-42 monomers (SPR-485, left), oligomers (SPR-488, middle) and fibrils (SPR-487, right).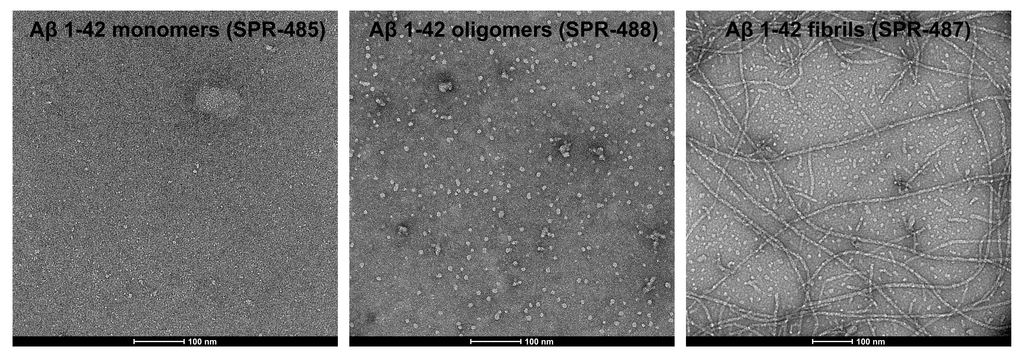
AFM of amyloid beta 1-42 monomers (SPR-485, left), oligomers (SPR-488, middle) and fibrils (SPR-487, right).
Toxicity
StressMarq’s Amyloid Beta 1-42 oligomers, pre-formed fibrils (PFFs) and monomeric peptide can be used for neurodegenerative disease research.
Amyloid Beta 1-42 Oligomers (catalog# SPR-488) and Amyloid Beta 1-42 PFFs (catalog# SPR-487) show a dose-dependent toxicity to primary rat cortical neurons, whereas our Amyloid Beta 1-42 Peptide (monomeric) (catalog# SPR-485) does not show toxicity.
The charts below show survival of rat primary cortical neurons 14 days after treatment with different concentrations of (A) monomers, (B) oligomers or (C) fibrils quantified by MAP2 positive neurons and expressed as a percentage of control. Fibrils and respective vehicle controls were initially sonicated in a Bioruptor. Test conditions were run in the same plate as untreated control and vehicle controls, which consisted of buffer without amyloid beta 1-42.
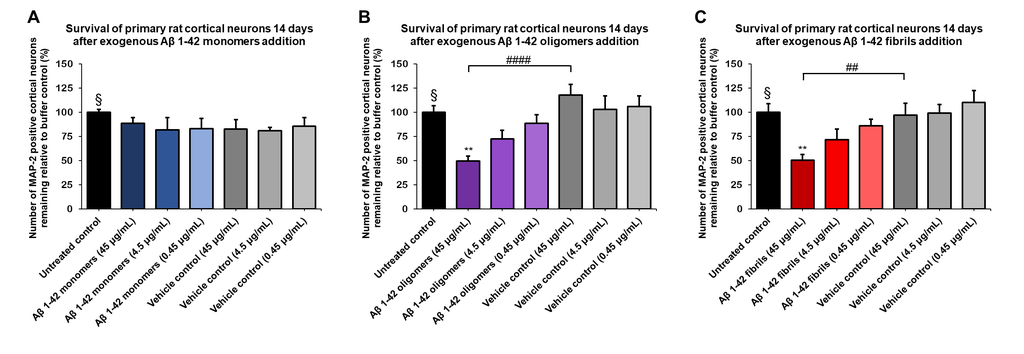
Vehicle controls consisted of buffer without amyloid beta 1-42. Sonication (Bioruptor) was only performed on fibrils and respective vehicle controls. Test conditions were run in the same plate as respective controls. Data expressed as mean +/- s.e.m. (n=6) and statistics determined by one-way ANOVA followed by Dunnett’s test; ** p<0.01 stats vs control; ## p<0.01, #### p<0.0001 stats vs vehicle control. § represents untreated control condition.
References
1. Stine et al. 2003. JBC. 278(13):11612-22. doi: 10.1074/jbc.M210207200
2. Ahmed et al. 2010. Nature Structural & Molecular Biology. 17(5):561-7. doi: 10.1038/nsmb.1799
3. Panza et al. 2019. Nat Rev Neurol. 15:73-88 https://doi.org/10.1038/s41582-018-0116-6
4. Shankar et al. 2008. Nat Med. 14(8):837-842. doi: 10.1038/nm1782
5. Chromy et al. 2003. Biochemistry. 42:12749-12760. doi: 10.1021/bi030029q
6. Kayed et al. 2003. Science. 300(5618): 486-489. doi: 10.1126/science.1079469
7. Want et al. 2016. JAMA Neurol. 73(9):1070-7. doi: 10.1001/jamaneurol.2016.2078
8. Kotzbauer et al. 2012. Arch Neurol. 69(10): 1326-1331. doi: 10.1001/archneurol.2012.1608
Scientific Publications Using StressMarq’s Amyloid Beta Proteins
- Chimeric antigen receptor macrophages target and resorb amyloid plaques. Kim, A.B. et al. JCI Insight. 2024.
- Catalog# SPR-485: Human Synthetic Amyloid Beta 1-42 Peptide (HFIP-treated)
- Catalog# SPR-488: Human Synthetic Amyloid Beta 1-42 Oligomers
- Catalog# SPR-492: Human Amyloid Beta Pyroglutamate 3-42 Pre-formed Fibrils
- α-Synuclein-dependent increases in PIP5K1γ drive inositol signaling to promote neurotoxicity. Horvath, J.D. et al. Cell Reports. 2023.
- Catalog# SPR-487: Human Synthetic Amyloid Beta 1-42 Pre-formed Fibrils
Supplemental Learning Materials
Technical Support Resources
- Protocols | Amyloid Beta
- Handling Instructions | Amyloid Beta
- Sonication Protocol | PFFs
- Frequently Asked Questions | Neuro Proteins
Selected Media from the StressMarq YouTube Channel
- Webinar | Tools for Alzheimer’s & Parkinson’s Research | Presented by Dr. Jacob McPhail, Lead R&D Scientist, StressMarq
- Open Theatre | Fibrillar & Oligomeric Constructs of Neurodegenerative Disease Asssociated Peptides | Presented by Dr. Ariel Louwrier, President & CEO, StressMarq
Selected Articles from the StressMarq Blog
- Amyloid Beta Fibrillar Constructs | Learn about our monomeric, fibrillar and oliogmeric amyloid beta constructs.
- Uncovering Links Between Amyloid Beta and Alpha Synuclein in Neurodegenerative Diseases | Recent evidence supports a synergistic relationship between amyloid beta & alpha synuclein in neurodegeneration.
- The Role of Amyloid Beta Oligomers in Alzheimer’s Disease | Amyloid beta oligomers are thought to be the major toxic species responsible for Alzheimer’s pathology.
- Amyloid Hypothesis vs Tau Hypothesis | Amyloid beta and tau aggregation are both thought to contribute to Alzheimer’s disease, but there is discourse as to which protein aggregates first.
Have a question about Amyloid Beta?
Featured Amyloid Beta Products