Targeting Tau Monomers: Potential for Therapeutic Interventions
Microtubule-associated protein tau, encoded by the MAPT gene, is expressed predominantly in neurons, where it has an essential role in many physiological processes. Abnormal hyperphosphorylation and aggregation of tau within neuronal cells is a classic hallmark of Alzheimer’s disease, Parkinson’s disease, and many other so-called tauopathies, highlighting the need to both understand and prevent or reverse this process. By targeting tau for therapeutic intervention, researchers hope to improve patient outcomes and develop effective treatments for neurodegenerative disorders that currently have no cure.
Why target tau monomers?
Under normal conditions, tau is monomeric, being expressed as six different isoforms (ranging from 352 – 441 amino acids in length) in the adult human brain1. These are involved in regulating microtubule assembly and stability, which is critical for axonal outgrowth and the transport of synaptic vesicles. However, under pathological conditions, hyperphosphorylated tau monomers dissociate from negatively-charged microtubules to aggregate firstly into oligomers, and then into neurofibrillary tangles (NFTs), subsequently leading to neuronal degeneration2,3.
Although tau pathology is not yet fully understood, several hypotheses have been proposed. One suggestion is that seed-competent tau monomers derived from naïve tau through a structural transition may trigger aggregation, indicating the therapeutic potential of preventing this conversion4. Another is that lysosomal stress may stimulate the release of seed-competent monomeric tau into the extracellular space, prompting researchers to consider the therapeutic utility of inhibiting lysosomal exocytosis5. It is also known that disease-associated mutations can destabilize the aggregation-prone VQIVYK motif within tau, which may therefore prove to be a valuable therapeutic target6.
How can tau monomers be used in aggregation assays?
Various models have been developed to investigate the process of tau aggregation, many of which rely on recombinant tau monomers to replicate aggregate assembly in vitro. A popular approach is to use aggregation inducers such as polyanions (e.g. heparin), fatty acids (e.g. arachidonic acid), or pre-formed tau filaments to seed aggregates from a pool of tau monomers, using the fluorescent probe Thioflavin S (that binds beta sheet-rich structures) to monitor aggregate formation in real time; this allows, for example, the aggregation rate of different tau isoforms, truncated tau domains, or mutant forms of the protein to be compared3. Other types of assay are designed to study the effects of over-expressing tau monomers in various cell lines, again using secondary methods such as Thioflavin S to track the aggregate production.
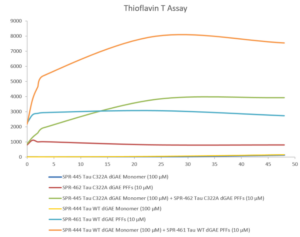
High-quality tau monomers for aggregation assays
We have developed a broad selection of reagents for studying tau and its role in neurodegenerative disease. These include our Active Human Recombinant Tau441 (2N4R), P301S mutant Protein Monomer that is expressed in either E. coli (SPR-327) or baculovirus (SPR-473), our Active Human Recombinant Tau441 (2N4R) Wild-Type Protein Monomer (SPR-479), our Active Human Recombinant Tau (K18), P301L mutant Protein Monomer (SPR-328), and our Active Human Recombinant Tau (K18) K280 Deletion Protein Monomer (SPR-476). These are complemented by our Active Human Recombinant Truncated Tau Fragment (AA297-391) Protein Monomers dGAE (SPR-444) and dGAE C322A (SPR-445), as well as our Active Mouse Recombinant Tau441 (2N4R), P301S mutant Protein Monomer (SPR-474). Many of these have been validated using Thioflavin T to monitor aggregation.
Thioflavin T is a fluorescent dye that binds to beta sheet-rich structures, such as those in tau fibrils. Upon binding, the emission spectrum of the dye experiences a red-shift and increased fluorescence intensity. Thioflavin T emission curves show increased fluorescence (correlated to tau aggregation) over time when truncated tau fragment (AA297-391) (dGAE C322A) monomer is combined with truncated tau fragment (AA297-391) (dGAE C322A) preformed fibrils (Type 1).
REFERENCES
- Tau‑mediated Neurodegeneration and Potential Implications in Diagnosis and Treatment of Alzheimer’s Disease, Wu XL et al, Chin Med J (Engl). 2017 Dec 20;130(24):2978-2990
- Phosphorylation and Dephosphorylation of Tau Protein During Synthetic Torpor, Luppi M et al, Front. Neuroanat., 06 June 2019
- Cell-based Models To Investigate Tau Aggregation, Lim S et al, Comput Struct Biotechnol J. 2014 Oct 2;12(20-21):7-13
- Inert and seed-competent tau monomers suggest structural origins of aggregation, Mirbaha H et al, Elife. 2018 Jul 10;7:e36584
- Seeding Activity-Based Detection Uncovers the Different Release Mechanisms of Seed-Competent Tau Versus Inert Tau via Lysosomal Exocytosis, Tanaka Y et al, Front Neurosci. 2019 Nov 21;13:1258
- Tau local structure shields an amyloid-forming motif and controls aggregation propensity, Chen D et al, Nat Commun. 2019 Jun 7;10(1):2493


Leave a Reply