Tau in Alzheimer’s Disease Therapeutics
Abnormal protein deposition is a common feature of many neurodegenerative disorders. Included among these, Alzheimer’s disease is characterized by the presence of extraneuronal amyloid beta (Aβ) plaques and intraneuronal deposits of the microtubule-associated protein tau in the brain. Since amyloid beta and tau were first identified, therapies aimed at treating Alzheimer’s disease have mainly targeted amyloid beta. However, toxicity and/or a failure to demonstrate efficacy in clinical trials has led to a shift in focus toward tau1. Moreover, because tau deposition has been shown to correlate more closely than amyloid beta deposition to cognitive impairment, it is possible that tau may actually be a more relevant therapeutic target2.
What are the approaches to targeting tau in Alzheimer’s disease?
The cognitive defects experienced by patients with Alzheimer’s disease are thought to result largely from neuronal axons becoming blocked by tau deposition, whereby the essential transport of biomolecules and organelles is impeded3. As such, therapies being developed to treat Alzheimer’s disease often target the underlying mechanisms of tau deposition, and include the following approaches:
Inhibiting tau kinases
Because the intraneuronal deposits characteristic of Alzheimer’s disease are composed mainly of hyperphosphorylated tau, many potential therapies are aimed at inhibiting the responsible kinases. CDK5, GSK3β and ERK2 have all been linked to aberrant tau phosphorylation, with ATP-competitive small molecule inhibitors of all three kinases proving able to modulate tau phosphorylation in vitro4.
Preventing tau aggregation
Blocking tau-tau interactions has significant therapeutic potential since the intraneuronal tau deposits found in the brains of Alzheimer’s disease patients form by self-propagation5. Various compounds have been shown to have an inhibitory effect on this process, with the phenothiazine dye methylene blue (Rember®) showing some degree of success in phase II clinical trials6,7. Structure-based design strategies have exploited the fact that the tau sequence segments VQIINK and VQIVYK drive its aggregation, with inhibitors based on the structure of the VQIINK segment being the more effective at preventing seeding8.
Promoting tau clearance
Another approach to target aberrant tau is to harness normal physiological mechanisms for removing misfolded or aggregated proteins. One example is to enhance tau degradation by the ubiquitin-proteasome system, a process that can be stimulated with inhibitors of HSP90 – a molecular chaperone involved in protein refolding9.
Immunotherapeutic approaches
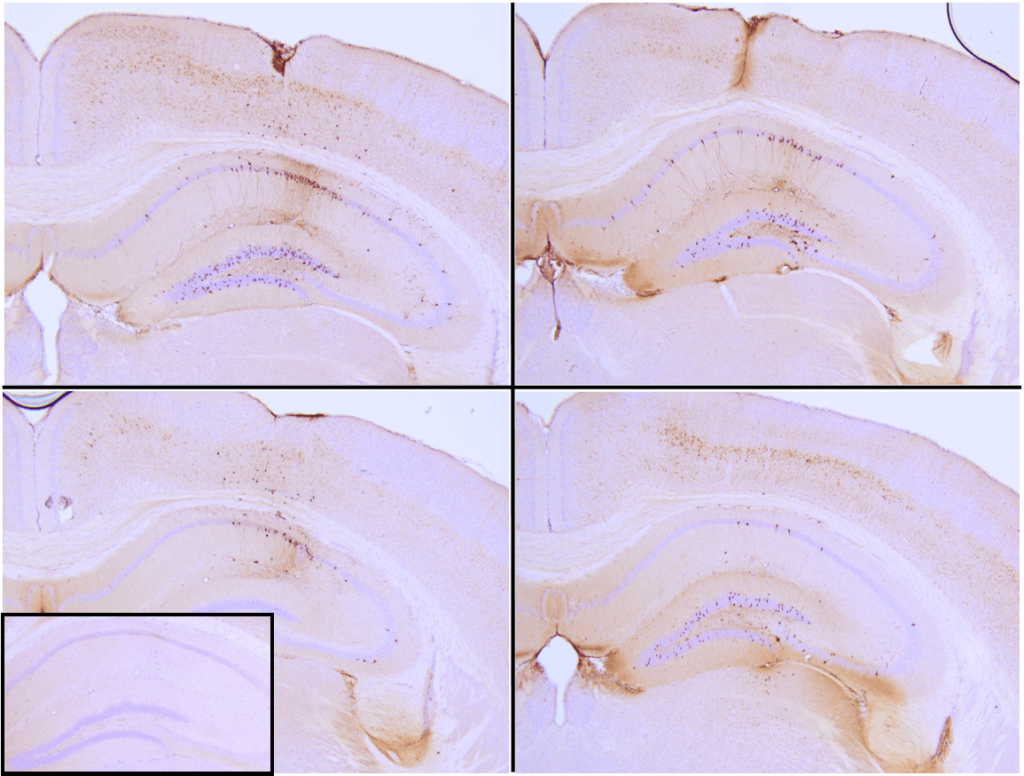
Figure 1: Immunohistochemical analysis of P301L mouse hippocampus injected with K18 P301L tau PFFs (SPR-330) shows seeding of tau pathology at injection site nine weeks post-injection. AT8 (pSer202/pThr205) tau antibody shows tangle-like inclusions. Inset: negative control. Experiments performed at reMYND N.V.
Both active and passive immunotherapies are being developed to target tau, with a main driver being the promise shown by the use of anti-amyloid beta antibodies to prevent cognitive deficiencies in transgenic mouse models3. Although a main difference between amyloid beta and tau is that tau is intraneuronal, one hypothesis proposes that anti-tau antibodies might function by preventing the spread of tau pathology between neurons. This theory is consistent with tau being detectable in the cerebrospinal fluid, where its presence represents a promising biomarker for Alzheimer’s disease10.
Supporting the study of tau and its role in Alzheimer’s disease
StressMarq offers a wide range of products to advance understanding of tau and amyloid beta to support researchers developing Alzheimer’s disease therapeutics. Our portfolio includes high-quality tau and amyloid beta proteins and antibodies, as well as our active tau preformed fibrils (PFFs) and filaments that can be used to seed the process of tau aggregation.
References
1) Tau-targeting therapies for Alzheimer disease, Congdon EE and Sigurdsson EM, Nat Rev Neurol. 2018 Jul;14(7):399-415
2) Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease, Brier MR et al, Sci Transl Med. 2016 May 11;8(338):338ra66
3) The Role of Tau in Neurodegenerative Diseases and Its Potential as a Therapeutic Target, Wolfe MS, Scientifica (Cairo). 2012;2012:796024
4) Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases, Mazanetz MP and Fischer PM, Nat Rev Drug Discov 2007 Jun;6(6):464-79
5) Tau-aggregation inhibitor therapy for Alzheimer’s disease, Wischik CM et al, Biochem Pharmacol. 2014 Apr 15;88(4):529-39
6) Advances in Tau-focused drug discovery for Alzheimer’s disease and related tauopathies, Brunden KR et al, Nat Rev Drug Discov. 2009 Oct;8(10):783-93
7) Drug candidates in clinical trials for Alzheimer’s disease, Hung SY and Fu WM, J Biomed Sci. 2017 Jul 19;24(1):47
8) Structure-based inhibitors of tau aggregation, Seidler PM et al, Nat Chem. 2018 Feb;10(2):170-176
9) Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species, Dickey CA et al, J Neurosci. 2006 Jun 28;26(26):6985-96
10) Cerebrospinal fluid and plasma biomarkers in Alzheimer disease, Blennow H et al, Nat Rev Neurol. 2010 Mar;6(3):131-44


Leave a Reply