Tau Post-Translational Modifications: An Overview of the Baculovirus Expression Vector System
For more than a decade, the biotechnology industry has produced recombinant proteins that have applications in medicine, scientific research, agriculture, and food production. These proteins are synthesized in heterologous systems as it is impossible to isolate them from natural sources. Recent advances in recombinant protein technology have made it possible to clone the DNA encoding a specific protein into an expression vector and express the protein in expression systems such as bacteria, yeast, insect, and mammalian cells1. Post-translational modifications (PTMs) play an important role in disease progression such as the tau PTMs in Alzheimer’s. The baculovirus expression vector system is the most popular technique to express proteins which require post-translational modifications2.

Test tubes and flasks at the CSIRO NCRIS Recombinant Protein Production Facility.CSIRO / CC BY (https://creativecommons.org/licenses/by/3.0)
What is Baculovirus?
Baculovirus is a large, enveloped virus with a double-stranded circular DNA of 80-180 kbp and belongs to Baculoviridae family. The pathogen infects only insects which made it a great biopesticide in crop fields in the 1940s. Later in the 1990s, scientists started to use baculoviruses as expression vectors for producing eukaryotic proteins in insect cell cultures.
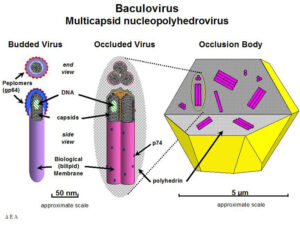
Diagram of nucleopolyhedrovirus virions.
Baculovirus expression vector system (BEVS)
Baculovirus vectors are used to insert desired gene and transfected into cultured insect cells to produce high levels of recombinant proteins. Autographa californica nuclear polyhedrosis virus (AcNPV) is the most widely used baculovirus and the Sf9 insect cells which are derived from the ovaries of the fall armyworm (Spodoptera frugiperda) are a suitable host for the baculovirus3.
In the very late phase of the viral replication, the polyhedrin and p10 genes are highly expressed but their proteins are not essential for the pathogenesis into a host insect cell. These genes can be replaced with the foreign gene of interest and the polyhedrin and p10 promoters can be used for its expression in cultured cells.
The gene of interest is cloned into a transfer vector and then the recombinant transfer vector and the wild-type AcNPV DNA are co-transfected into insect cell cultures (Sf9). Due to homologous recombination, the foreign gene is transferred to the AcNPV DNA and the recombinant virus replicates in the insect cells, leading to highly efficient production of the desired protein3,4.
Advantages of baculovirus expression vector system (BEVS)
Protein production with baculovirus-infected insect cells is considered the most efficient method for high- level expression of heterologous proteins. This is because:
- The large baculovirus genome allows for large insertions which makes it ideal for expressing large protein complexes.
- Baculovirus is non- pathogenic to mammals and plants which makes the system safe and has low cost compared to mammalian systems.
- Many recombinant proteins are produced in soluble form, are properly folded and contain the appropriate post-translational modifications5.
BEVS has been used widely in vaccine development and as gene therapy vectors preparation of cell signaling proteins. The proteins expressed from this eukaryotic system are used in research, vaccines, and diagnostics6.
Phosphorylated Tau in transfected Sf9 cells
Tau phosphorylation has received great attention as the protein aggregates when it is highly phosphorylated in Alzheimer’s disease. One of the major advantages of the baculovirus expression system is the production of large quantities of post-translationally modified heterologous proteins. The insect cell lines Sf9 are capable of introducing similar post-translational modifications to mammalian cells in contrast to prokaryotic expression systems. Tau phosphorylation in transfected Sf9 cells is surprisingly similar to that of neuronal cells transfected with tau which suggests that the Sf9 cell is a good model for studying the role of tau phosphorylation in neurite outgrowth7,8.
StressMarq Biosciences offers several Tau Pre-formed Fibrils (PFFs) that have been expressed in Baculovirus/Sf9, including catalog# SPR-498, catalog# SPR- 471. We also offer monomers, including catalog# SPR-496 and catalog# SPR-473. These products are post-translationally modified due to the baculovirus/SF9 insect cells expression system. For a complete list of StressMarq’s tau proteins, please see our tau page.
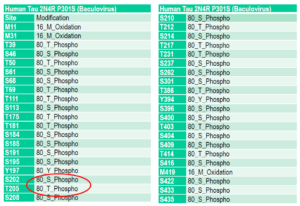
Mass spectrometry analysis of Tau441 (2N4R) P301S mutant pre-formed fibrils (PFFs) (SPR-471) expressed in baculovirus. Mass spectrometry analysis shows phosphorylation at sites including threonine 181, serine 202, and threonine 205.
REFERENCES
- Recombinant DNA technology in eukaryotes. Griffiths AJF et al. (2000) An Introduction to Genetic Analysis, 7th Edition.
- Use of baculovirus expression system for generation of virus-like particles: successes and challenges. Liu F. et al. (2013) Protein Expr Purif. 90(2):104‐116.
- Opportunities and Challenges for the Baculovirus Expression System. Van Oers M.M. (2011) J Invertebr Pathol. 107: S3‐S15.
- Overview of the Baculovirus Expression System. Murphy, C.I. and Piwnica‐Worms, H. (2000), Current Protocols in Neuroscience, 10: 4.18.1-4.18.4.
- FlexiBAC: a versatile, open-source baculovirus vector system for protein expression, secretion, and proteolytic processing. Lemaitre, R.P. et al. (2019) BMC Biotechnol 19: 20.
- The Baculovirus Expression Vector System: A Commercial Manufacturing Platform for Viral Vaccines and Gene Therapy Vectors. Felberbaum RS. (2015) Biotechnol J. 10(5):702‐714.
- The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains. Biernat J. et al. (1999) Mol Biol Cell.10(3):727‐740.
- Oligomer formation of tau protein hyperphosphorylated in cells. Tepper K. et al. (2014) J Biol. Chem. 289(49): 34389–34407.


Leave a Reply