Tau Protein and its Role in Alzheimer’s Disease
Alzheimer’s disease is named after the renowned German neuropathologist and psychiatrist, Alois Alzheimer, who, in 1906, observed abnormal clumps and tangled fibrous bundles throughout the brain of a patient who had died following an unusual mental illness. These have since been identified as amyloid plaques and neurofibrillary tangles and are now widely recognized as the most common hallmarks of Alzheimer’s disease. The microtubule-associated protein tau is a major component of the neurofibrillary tangles, suggesting an important role for tau in Alzheimer’s disease pathogenesis. As the incidence of Alzheimer’s disease increases with an ageing population, research efforts to understand tau pathology are essential to developing life-changing therapeutics.
How does tau aggregation lead to neurodegeneration in Alzheimer’s Disease?
Tau is encoded by the MAPT gene, which undergoes alternative splicing to produce six isoforms ranging from 352-441 amino acids in length. Each isoform consists of three domains – a projection domain, a proline-rich domain, and an assembly domain – which vary according to the number of N-terminal inserts within the projection domain (0, 1, or 2), and the number of C-terminal repeats within the assembly domain (3 or 4)1. Under normal conditions, the different domains perform distinct roles in promoting the assembly and stability of microtubules within neuronal axons. However, during neurodegenerative conditions such as Alzheimer’s disease, this vital function is lost.
Several molecular mechanisms have been proposed to underlie tau pathology. These include phosphorylation, truncation, acetylation, ubiquitination, and sumoylation, of which phosphorylation is the most widely studied to date2. Multiple papers have been published linking hyperphosphorylation of tau to protein misfolding and aggregate formation, many of which implicate specific phosphorylation sites, and it has been suggested that this process is particularly detrimental to neurons since their axonal projections are narrow and easily blocked. As the tau aggregates interfere with normal physiological transport of biomolecules and organelles, neurons can degenerate from the axon to the cell body. Indeed, the unique biology of neurons is a likely reason that abnormal tau aggregation underlies not only Alzheimer’s disease but many other neurodegenerative conditions, collectively referred to as tauopathies3.
Researching tau aggregation
Although researching tau aggregation is extremely challenging, many studies are ongoing to try and establish exactly how this leads to neuronal cell death4. It has been proposed that tau pathology may be secondary to the accumulation of amyloid plaques, yet there is growing evidence to indicate that tau aggregates are also involved in many processes that are independent of amyloid plaque formation1. Understanding these activities and discovering how tau might be manipulated to prevent its aggregation remains key to designing effective treatment strategies for Alzheimer’s disease5,6.
Supporting the study of tau aggregation in Alzheimer’s Disease
StressMarq’s portfolio of high-quality research reagents contains many products designed to advance understanding of tau aggregation in Alzheimer’s disease. These include active human recombinant tau (K18) P301L mutant protein pre-formed fibrils (SPR-330), active human recombinant truncated tau fragment (AA297-391) (dGAE) protein preformed fibrils (SPR-461), human recombinant tau441 (2N4R) P301S mutant protein filaments (SPR-463), and active human recombinant tau (K18) K280 deletion protein pre-formed fibrils (SPR-477). These can be used to induce endogenous tau phosphorylation and aggregation, leading to disease pathology in vivo.
TEM of active human recombinant truncated tau fragment (AA297-391) (dGAE) protein preformed fibrils (Type 1) (SPR-461).
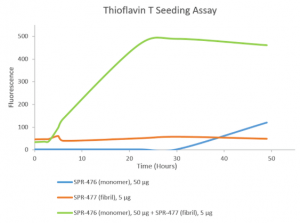
Thioflavin T is a fluorescent dye that binds to beta sheet-rich structures, such as those in tau fibrils. Upon binding, the emission spectrum of the dye experiences a red-shift and increased fluorescence intensity. Thioflavin T emission curves show increased fluorescence (correlated to tau aggregation) over time when tau monomers (SPR-476) are combined with tau fibrils (SPR-477).
REFERENCES
- Tau‑mediated Neurodegeneration and Potential Implications in Diagnosis and Treatment of Alzheimer’s Disease, Wu XL et al, Chin Med J (Engl). 2017 Dec 20;130(24):2978-2990
- Phosphorylation of different tau sites during progression of Alzheimer’s disease, Neddens J et al, Acta Neuropathol Commun. 2018 Jun 29;6(1):52
- The Role of Tau in Neurodegenerative Diseases and Its Potential as a Therapeutic Target, Wolfe MS, Scientifica (Cairo). 2012;2012:796024
- Tau protein in neurodegenerative diseases – a review, Pîrşcoveanu DFV et al, Rom J Morphol Embryol. 2017;58(4):1141-1150
- What renders TAU toxic, Götz J et al, Front Neurol. 2013 Jun 10;4:72
- Roles of tau protein in health and disease, Guo T et al, Acta Neuropathol. 2017 May;133(5):665-704


Leave a Reply