Properties
| Storage Buffer | 50mM Tris/HCl pH7.5, 0.3M NaCl, 10% glycerol, 0.1mM EDTA |
| Storage Temperature | -20ºC |
| Shipping Temperature | Blue Ice or 4ºC |
| Purification | Endotoxin-free, Multi-Step Purified |
| Cite This Product | Human Recombinant HSP70 Protein (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-117) |
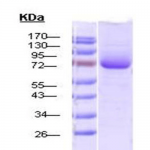
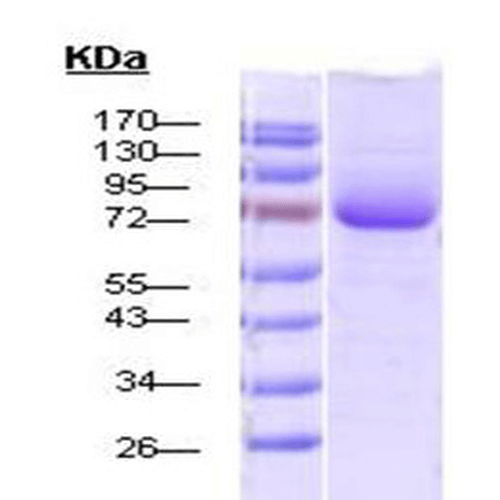
| Certificate of Analysis | This product has been certified >90% pure using SDS-PAGE analysis. The protein tested positive for ATPase activity using a Malachite Green assay. |
Biological Description
| Alternative Names | HSPA1A, HSPA1B, HSPA1, HSP70, HSP70-1, HSP70.1, HSP70-2, HSP72, HSP73, HSX70, Heat shock 70 kDa protein 1A, Heat shock 70 kDa protein 1B |
| Research Areas | Cancer, Heat Shock |
| Cellular Localization | Cytoplasm |
| Accession Number | M11717 |
| Gene ID | 3303 |
| Swiss Prot | P0DMV8/P0DMV9 |
| Scientific Background |
HSP70 is a highly inducible molecular chaperone that plays a pivotal role in protein quality control across all major cellular compartments. In the brain, HSP70 is essential for preventing the aggregation of misfolded proteins, a key pathological feature of neurodegenerative diseases. HSP70 binds to nascent and partially folded polypeptides, stabilizing them and facilitating proper folding or directing them toward degradation pathways. This function is particularly critical in conditions such as Alzheimer’s, Parkinson’s, and ALS, where protein aggregation and impaired proteostasis drive neuronal dysfunction and cell death. The chaperone activity of HSP70 is regulated by ATP binding and hydrolysis, which triggers conformational changes that control substrate binding and release. Its ability to recognize hydrophobic regions of unfolded proteins enables it to suppress aggregation and promote cellular recovery from stress. Therapeutic strategies aimed at enhancing HSP70 expression or activity are under investigation for their potential to reduce neurotoxicity, improve protein clearance, and slow disease progression in neurodegenerative disorders. |
| References |
1. Zho J. (1998) Cell. 94: 471-480. 2. Boorstein W. R., Ziegelhoffer T. & Craig E. A. (1993) J. Mol. Evol.38(1): 1-17. 3. Rothman J. (1989) Cell. 59: 591 -601. 4. DeLuca-Flaherty et al. (1990) Cell. 62: 875-887. 5. Bork P., Sander C. & Valencia A. (1992) Proc. Natl Acad. Sci. USA. 89: 7290-7294. 6. Fink A.L. (1999) Physiol. Rev. 79: 425-449. |





















StressMarq Biosciences :
Based on validation through cited publications.