| Product Name | Ubiquitin Detection Kit (Discontinued) |
| Description |
Capture and detection of ubiquitinated proteins |
| Species Reactivity | Species Independent |
| Platform | Spin Column |
| Sample Types | Cell lysates, Tissue |
| Assay Type | Binding Matrix/Quantitative WB (Western Blot) |
| Utility | Detection kit used to capture, detect, identify and characterise ubiquitinated proteins and free chains from samples. |
| Incubation Time | 1 hour |
| Number of Samples | 20 samples |
| Other Resources | Kit Booklet , MSDS |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | 4ºC | |||||||||||||||
| Shipping Temperature | Blue Ice | |||||||||||||||
| Product Type | Detection Kits | |||||||||||||||
| Assay Overview | The Ubiquitin Detection kit facilitates the fast, effective capture and detection of ubiquitinated proteins from biological samples. The kit utilises a high capacity, high specificity ubiquitin binding matrix together with an easy-to-use purification system for less ‘hands on time’ and superior performance. Allows purification of mono- and poly-ubiquitinated proteins, independent of chain linkage or length, but not free ubiquitin. Highly adaptable, compatible with samples from a wide range of species and with a broad range of lysis buffers. Analysis by Western blotting or proteomic methods enables identification and assessment of ubiquitinated proteins of interest. Kit contains sufficient ubiquitin matrix to perform up to 20 assays. | |||||||||||||||
| Kit Overview |
|
|||||||||||||||
| Cite This Product | Ubiquitin Detection Kit (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SKT-131) |
Biological Description
| Alternative Names | Polyubiquitin B Detection Kit, RPS27A Detection Kit, UBA52 Detection Kit, UBB Detection Kit, UBC Detection Kit, ubiquitin B Detection Kit |
| Research Areas | Apoptosis, Autophagy, Cancer, Cardiovascular System, Cell Signaling, Neurodegeneration, Neuroscience, Post-translational Modifications, Ubiquitination |
| Scientific Background |
The covalent attachment of ubiquitin to proteins (ubiquitination) plays a fundamental role in the regulation of cellular function through biological events including cell cycle, differentiation, immune responses, DNA repair, chromatin structure, transcription, signal transduction, endocytosis, apoptosis and degradation by the proteasome, autophagy and lysosome systems. As such ubiquitin signalling and the processes it mediates are essential for the normal functioning of cells and its dysfunction has been implicated in wide range of diseases including cancer, neurodegeneration, cardiovascular and metabolic disorders (1,2). The type of ubiquitin modification, (monoubiquitin, multiubiquitin, polyubiquitin), substrate protein lysine residue(s) modified and, in the case of polyubiquitination, the chain length and lysine linkage type control the function and fate of ubiquitinated proteins. In addition all ubiquitin mediated pathways also utilise specific ubiquitin receptors to facilitate their regulation (3,4). Ubiquitination is achieved through three enzymatic steps. In an ATP-dependent process, the ubiquitin E1 activating enzyme catalyses the formation of a reactive thioester bond with ubiquitin, followed by its subsequent transfer to the active site cysteine of a ubiquitin E2 carrier protein. The selectivity of the ubiquitin cascade for a particular substrate protein relies on the interaction between the E2 conjugating enzyme (of which a cell contains relatively few) and an ubiquitin E3 ligase , of which over 600 have been identified to date. The specific E2-E3 pair required for ubiquitination of a particular substrate protein in vivo may also control the type, point and length / linkage (polyubiquitin) of the ubiquitin modification (5). |
| References |
1. Fulda, S., Rajalingam, K. & Dikic, I. EMBO molecular medicine 4, 545–56 (2012). 2. Shaid, S., Brandts, C. H., Serve, H. & Dikic, I. Cell death and differentiation 20, 21–30 (2013). 3. Husnjak, K. & Dikic, I. Annual review of biochemistry 81, 291–322 (2012). 4. Komander, D. & Rape, M. Annual Review of Biochemistry 81, 203–229 (2012). 5. Spasser, L. & Brik, A. Angewandte Chemie (International ed. in English) 51, 6840–62 (2012). |

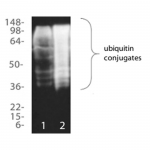
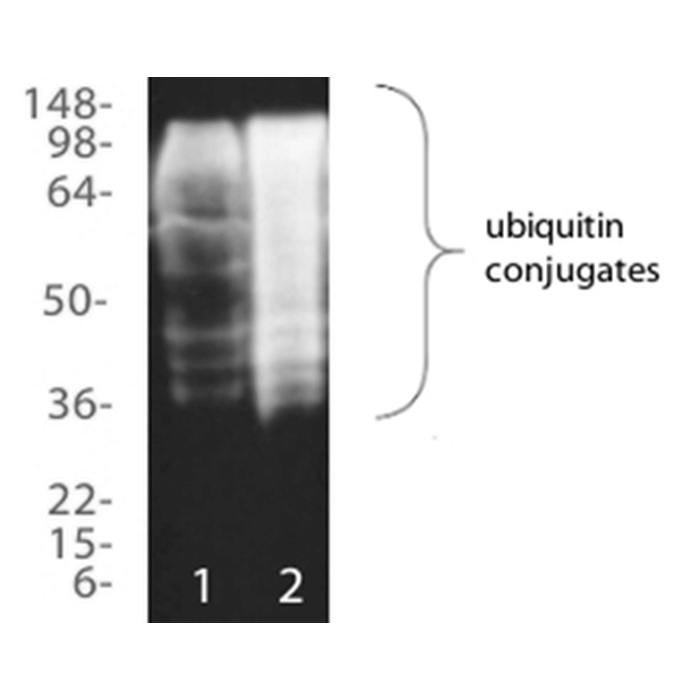
Reviews
There are no reviews yet.