| Product Name | Cortisone CLIA Kit (Discontinued) |
| Description |
Chemiluminescent measurement of cortisone |
| Species Reactivity | Species Independent |
| Platform | Microplate |
| Sample Types | Dried Fecal Samples, Plasma, Saliva, Serum, Tissue Culture Media, Urine |
| Detection Method | Chemiluminescent Assay |
| Assay Type | Sandwich CLIA (Chemiluminescent Immunoassay) |
| Utility | CLIA kit used to quantitatively measure the cortisone present in samples. |
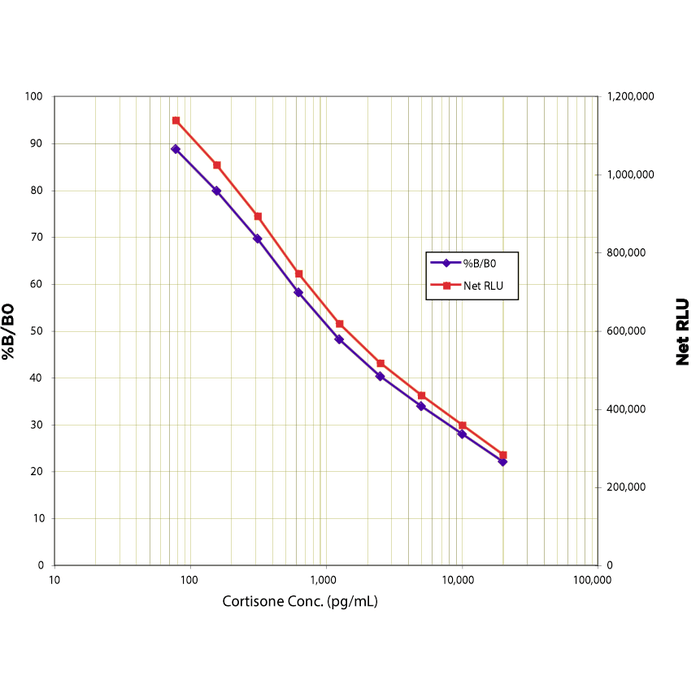
| Sensitivity | 10.6 pg/ml |
| Assay Range | 78.1 - 20,000 pg/ml |
| Precision | Intra Assay Precision: Three human samples were diluted with Assay Buffer and run in replicates of 20 in an assay. The mean and precision of the calculated Cortisone concentrations were: Sample 1- 7902.0 pg/mL, 7.9% CV Sample 2- 596.1 pg/mL, 5.7% CV Sample 3- 234.0 pg/mL, 10.1% CV Inter Assay Precision: Three human samples were diluted with Assay Buffer and run in duplicates in thirteen assays run over multiple days by three operators. The mean and precision of the calculated Cortisone concentrations were: Sample 1- 7904.0 pg/mL, 10.0% CV Sample 2- 662.7 pg/mL, 11.1% CV Sample 3- 262.9 pg/mL, 12.9% CV |
| Incubation Time | 2 hours |
| Number of Samples | 37 samples in duplicate |
| Other Resources | Kit Booklet , MSDS , Steroid Solid Extraction Protocol , Saliva Sample Handling Instructions |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | 4ºC | ||||||||||||||||||||||||||||||||||||
| Shipping Temperature | Blue Ice | ||||||||||||||||||||||||||||||||||||
| Assay Overview | The Cortisone CLIA kit is designed to quantitatively measure Cortisone present in extracted dried fecal samples, urine, saliva, and serum samples. This kit measures total cortisone in serum and plasma and in extracted fecal samples. A cortisone standard is provided to generate a standard curve for the assay and all samples should be read off the standard curve. Standards or diluted samples are pipetted into a white microtiter plate coated with an antibody to capture rabbit antibodies. A cortisone-peroxidase conjugate is added to the standards and samples in the wells. The binding reaction is initiated by the addition of a polyclonal antibody to cortisone to each well. After a two hour incubation the plate is washed and the chemiluminescent substrate is added. The substrate reacts with the bound cortisone-peroxidase conjugate to produce light. The generated light is detected in a microtiter plate reader capable of reading luminescence. The concentration of the cortisone in the sample is calculated, after making suitable correction for the dilution of the sample, using software available with most plate readers. | ||||||||||||||||||||||||||||||||||||
| Kit Overview |
|
||||||||||||||||||||||||||||||||||||
| Cite This Product | Cortisone CLIA Kit (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SKT-206) |
Biological Description
| Alternative Names | (8S,9S,10R,13S,14S,17R)-17-Hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthrene-3,11-dione CLIA Kit |
| Research Areas | Cell Signaling |
| Scientific Background | Cortisone (C21H28O5, Kendall’s Compound ‘E’) was identified by Mason, Myers and Kendall in 1936 as Compound E extracted from bovine suprarenal gland tissue that had the qualitative but not quantitative activity of cortin. The presence of multiple cortin-like compounds led the authors to speculate that the study of Compound E would reveal the nature of cortin (1). Compound E is now called cortisone and the more active Compound F, cortisol, and the concentrations of these two glucocorticoids vary due to the activity of two 11ß-hydroxysteroid dehydrogenases (11-HSD) (2,3). While most tissues have the ability to express either enzyme, 11ß-HSD1 is found primarily in the liver where it converts cortisone to cortisol while 11ß-HSD2 is found in tissues such as the kidney where cortisol receptor binding is required. 11ß-HSD2 deactivates cortisol to cortisone, prohibiting receptor activation. This glucocorticoid “shuttle” helps to initiate and regulate the anti-inflammatory response, making cortisone one of the modern “wonder drugs”. onitoring the ratio of cortisone:cortisol has applications in diabetes, obesity, metabolic syndrome, osteoporosis, and chronic fatigue syndrome in addition to adrenal diseases (4-7). Cortisone and cortisol concentrations exhibit a predictable diurnal pattern and can be measured in extracted dried feces, or in serum, plasma, saliva and urine. A recent publication (8) has suggested that salivary cortisone is a good surrogate marker for serum cortisol. |
| References |
1. Mason, HL, et al. J. Biol. Chem., 1936 116:267-276. 2. Mason, HL, et. al. J. Biol. Chem., 1938 124:459-474. 3. Hillier, SG. “Diamonds are Forever: the Cortisone Legacy” J. Endo., 2007 195:1-6. 4. van Raalte, DH, et al. Eur. J. Clin. Invest. 2009 39(2):81-93. 5. Pierotti, S, et al. J. Steroid Biochem. Mol. Biol. 2008 108(3-5):292-9. 6. Hadoke, PWF, et al. Br. J. Pharmacol. 2009 156:689-712. 7. Jerkes, WK, et al. J. Psychosomatic Res. 2006 60:145-153. 8. Perogamvros, I, et al. J Clin. Endocrin. Metab. 2010 August 4 (Epub ahead of print). |
Product Images
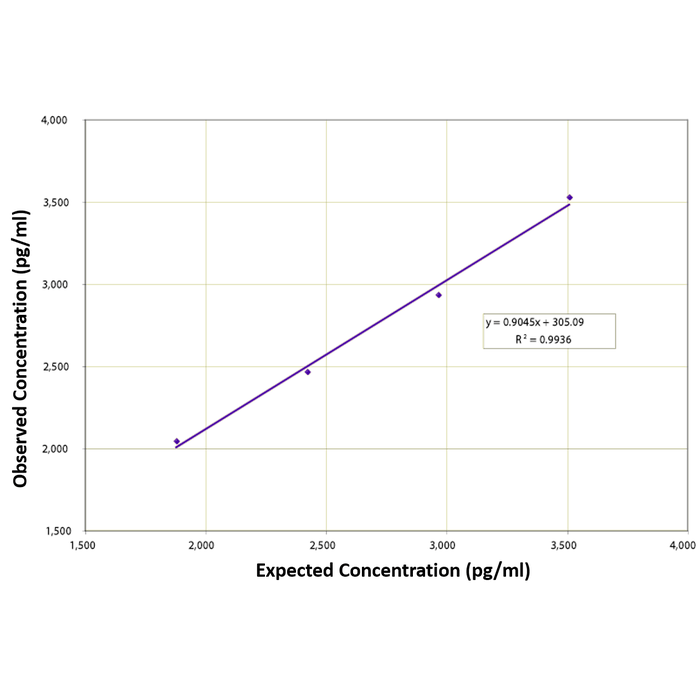
Linearity was determined by taking two serum samples treated with Dissociation Reagent and diluted 1:50 with Assay Buffer, one with a low diluted cortisone level of 1,336 pg/mL and one with a higher diluted level of 4,055 pg/mL, and mixing them. The measured concentrations were compared to the expected values based on the ratios used.



Reviews
There are no reviews yet.