| Product Name | Alpha-B Crystallin ELISA Kit |
| Description |
Colorimetric detection of Alpha B Crystallin |
| Species Reactivity | Human |
| Platform | Microplate |
| Sample Types | Cell lysates, Serum, Tissue |
| Detection Method | Colorimetric Assay |
| Assay Type | Sandwich ELISA (Enzyme-linked Immunosorbent Assay) |
| Utility | ELISA kit used to quantitate alpha B crystallin concentration in samples. |
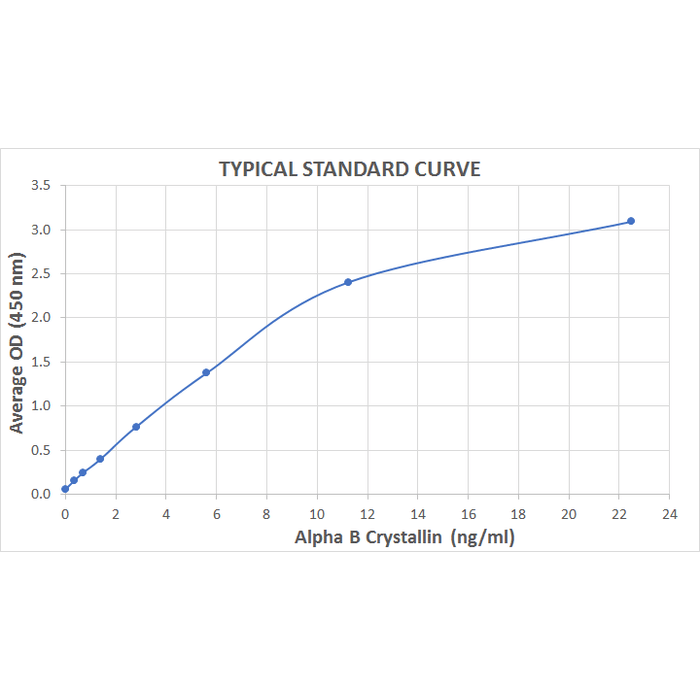
| Sensitivity | 0.009 ng/ml |
| Assay Range | 0.352 - 22.5 ng/ml |
| Incubation Time | 30 minutes |
| Number of Samples | 40 samples in duplicate |
| Other Resources | Kit Booklet Lot No. SA847285 , Kit Booklet Lot No. SA222909 , Kit Booklet Lot No. SA388800 , Kit booklet Lot No. SA287484 , Kit Booklet , MSDS |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | 4ºC and -20ºC | ||||||||||||||||||||||||||||||||||||
| Shipping Temperature | Blue Ice | ||||||||||||||||||||||||||||||||||||
| Product Type | ELISA Kits | ||||||||||||||||||||||||||||||||||||
| Assay Overview | 1. Prepare Standard and samples in Standard and Sample Diluent. 2. Add 100 µL of Standard or sample to appropriate wells. 3. Cover plate with Plate Sealer and incubate at room temperature (20-25°C) for 1 hour. 4. Wash plate four times with 1X Wash Buffer. 5. Add 100 µL of Biotinylated Antibody Working Solution to each well. 6. Cover plate with Plate Sealer and incubate at room temperature for 1 hour. 7. Wash plate four times with 1X Wash Buffer. 8. Add 100 µL of Streptavidin-HRP Working Solution to each well. 9. Cover plate with Plate Sealer and incubate at room temperature for 30 minutes. 10. Wash plate four times with 1X Wash Buffer. 11. Add 100 µL of TMB Substrate to each well. 12. Develop the plate in the dark at room temperature for 30 minutes. 13. Stop reaction by adding 100 µL of Stop Solution to each well. 14. Measure absorbance on a plate reader at 450 nm. | ||||||||||||||||||||||||||||||||||||
| Kit Overview |
|
||||||||||||||||||||||||||||||||||||
| Cite This Product | Alpha B Crystallin ELISA Kit (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SKT-123) |
Biological Description
| Alternative Names | AACRYA, CRYA2, CRYAB, CTPP2, HSPB5, NY Ren 27 antigen, alpha crystallin B |
| Scientific Background |
Alpha B-crystallin (CRYAB) is a small heat shock protein originally identified as a structural component of the vertebrate eye lens. Beyond its role in maintaining lens transparency, CRYAB functions as a molecular chaperone, preventing protein aggregation under stress conditions. It shares structural and functional similarities with other small heat shock proteins such as HSP25 and HSP27. Importantly, alpha B-crystallin is expressed in a wide range of tissues, including the central nervous system, where it plays a critical role in cellular protection and protein homeostasis. In the brain, CRYAB is upregulated in response to oxidative stress, inflammation, and protein misfolding—hallmarks of neurodegenerative diseases. Emerging research highlights CRYAB’s involvement in neurological disorders such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and Alexander disease. Its neuroprotective functions include inhibition of apoptosis via caspase suppression, stabilization of cytoskeletal elements, and interaction with key cellular components such as membrane proteins, nuclear proteins, and DNA. Due to its stress-inducible nature and anti-aggregation properties, alpha B-crystallin is increasingly recognized as a potential biomarker and therapeutic target in neurodegeneration. Its ability to modulate protein folding and prevent toxic aggregate formation positions it at the forefront of neuroscience research focused on proteinopathies. |
| References |
1. Merck K.B., et al. (1993) J Biol Chem. 268: 1046-1052. 2. Horwitz J. (1992) Proc Natl Acad Sci USA. 89(21):10449-10453. 3. Cobb B.A. and Petrash J.M. (2002) Biochemistry. 41:483-490 4. Horwitz J. (2003) Exp Eye Res. 76: 145-153. 5. Bullard B., et al. (2004) J Biol Chem. 279: 7917-7924. 6. Gangalum R.K., Schibler M.J. and Bhat S.P. (2004) J Biol Chem. 279: 43374.43377. 7. Maddala R. and Rao V.P. (2005) Exp Cell Res. 306: 203-215. 8. Yaung J., et al. (2007) Molecular Vision 13: 566-577. 9. Wilhelmus M.M.M., et al. (2006) Neuropathology and Applied Neurobiology. 32, 119–130. 10. Stege G.J.J., et al (1999) Biochemical and Biophysical Research Communications. 262, 152–156. 11. Outeiro T.F., et al (2006) Biochem Biophys Res Commun. December 22; 351(3): 631–638. 12. Rekas A., et al (2004) J. Mol. Biol. 340, 1167–118. 13. Yerbury J.J., et al (2013) Cell Stress and Chaperone. Mar;18(2):251-7. 14. Marino M., et al (2015) Neurobiology of Aging 36, 492-504. |


StressMarq Biosciences :
Based on validation through cited publications.