| Product Name | HSP90 alpha ELISA Kit |
| Description |
Colorimetric detection of HSP90 Alpha |
| Species Reactivity | Avian (Zebra finch), Goat, Human |
| Platform | Microplate |
| Sample Types | Cell lysates, Serum, Tissue, Whole Blood |
| Detection Method | Colorimetric Assay |
| Assay Type | Sandwich ELISA (Enzyme-linked Immunosorbent Assay) |
| Utility | ELISA kit used to quantitate HSP90 alpha concentration in samples. |
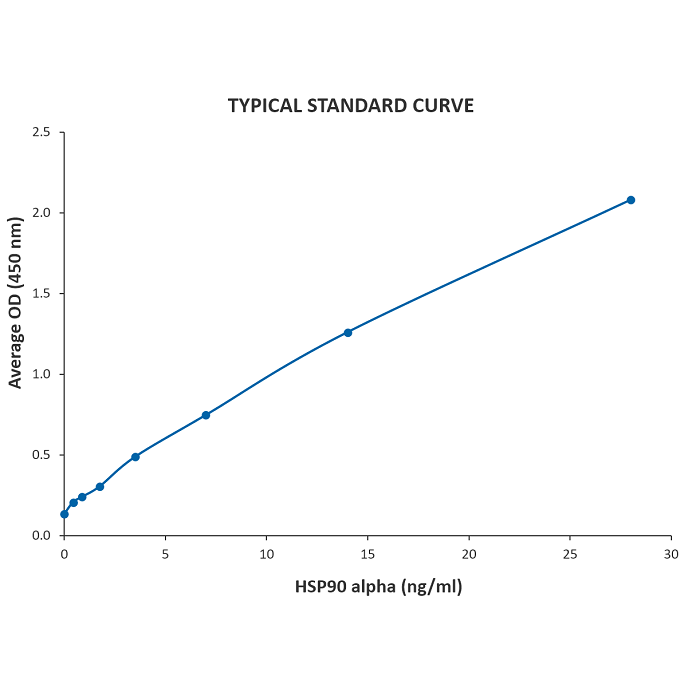
| Sensitivity | 0.117 ng/ml |
| Assay Range | 0.44 - 28 ng/ml |
| Incubation Time | 30 minutes |
| Number of Samples | 40 samples in duplicate |
| Other Resources | Kit Booklet Lot No. SH531948 , Kit Booklet Lot No. SH187452 , Kit Booklet Lot No. SH388280 , Kit Booklet Lot No. KH387128 , MSDS |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | 4ºC and -20ºC | ||||||||||||||||||||||||||||||||||||
| Shipping Temperature | Blue Ice | ||||||||||||||||||||||||||||||||||||
| Product Type | ELISA Kits | ||||||||||||||||||||||||||||||||||||
| Assay Overview | 1. Prepare Standard and samples in Standard and Sample Diluent. 2. Add 100 µL of Standard or sample to appropriate wells. 3. Cover plate with Plate Sealer and incubate at 37°C for 1 hour. 4. Wash plate four times with 1X Wash Buffer. 5. Add 100 µL of Biotinylated Antibody Working Solution to each well. 6. Cover plate with Plate Sealer and incubate at room temperature, 20-25 °C for 1 hour. 7. Wash plate four times with 1X Wash Buffer. 8. Add 100 µL of Streptavidin-HRP Working Solution to each well. 9. Cover plate with Plate Sealer and incubate at room temperature for 30 minutes. 10. Wash plate four times with 1X Wash Buffer. 11. Add 100 µL of TMB Substrate to each well. 12. Develop the plate in the dark at room temperature for 30 minutes. 13. Stop reaction by adding 100 µL of Stop Solution to each well. 14. Measure absorbance on a plate reader at 450 nm. | ||||||||||||||||||||||||||||||||||||
| Kit Overview |
|
||||||||||||||||||||||||||||||||||||
| Cite This Product | HSP90 alpha ELISA Kit (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SKT-107) |
Biological Description
| Alternative Names | HSP86, HSP89A, HSP90A, HSP90AA1, HSP90alpha, HSPC1, HSPCA, HsoCAL3 |
| Research Areas | Cancer, Cell Signaling, Chaperone Proteins, Heat Shock, Protein Trafficking, Tumor Biomarkers |
| Scientific Background |
Heat Shock Protein 90 (HSP90) is a highly conserved molecular chaperone essential for maintaining protein homeostasis across all eukaryotic cells. Although classified as a stress protein, HSP90 is abundantly expressed even under non-stress conditions, comprising up to 2% of cytosolic protein. Its core functions include the folding, maturation, stabilization, and trafficking of a wide range of client proteins. HSP90 plays a pivotal role in regulating key signaling molecules such as kinases (v-Src, Wee1, c-Raf), transcription factors (p53), steroid receptors, and enzymes like telomerase and viral polymerases. These interactions are mediated through ATP-dependent conformational changes and co-chaperone complexes involving Cdc37, p23, and immunophilin-like proteins, which protect client proteins from proteasomal degradation. In neuroscience, HSP90 is increasingly recognized for its role in modulating protein misfolding and aggregation—central features of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s. Alterations in HSP90 expression or function can disrupt neuronal signaling and impair the activity of neuroprotective proteins, contributing to disease progression. Pharmacological inhibitors of HSP90, including geldanamycin and radicicol, are being explored as therapeutic agents to restore proteostasis and reduce toxic protein accumulation in neurodegenerative models. |
| References |
1. Arlander S.J.H., et al. (2003) J Biol Chem. 278: 52572-52577. 2. Pearl H., et al. (2001) Adv Protein Chem. 59: 157-186. 3. Neckers L, et al. (2002) Trends Mol Med. 8:S55-S61. 4. Pratt W., Toft D. (2003) Exp Biol Med. 228:111-133. 5. Pratt W., Toft D. (1997) Endocr Rev. 18: 306-360. 6. Pratt W.B. (1998) Proc Soc Exptl Biol Med. 217: 420-434. 7. Whitesell L., et al. (1994) Proc Natl Acad Sci USA. 91: 8324- 8328. |

StressMarq Biosciences :
Based on validation through cited publications.