| Product Name | Prostaglandin E2 EIA Kit (Discontinued) |
| Description |
Quantitative colorimetric measurement of PGE2 |
| Species Reactivity | Species Independent |
| Platform | Microplate |
| Sample Types | EDTA Plasma, Heparin Plasma, Saliva, Serum, Tissue Culture Media, Urine |
| Detection Method | Colorimetric Assay |
| Assay Type | Sandwich EIA (Enzyme Immunoassay) |
| Utility | EIA kit used to quantitatively measure PGE2 present in samples. |
| Sensitivity | 3.07 pg/ml |
| Assay Range | HS: 1.953 - 500 pg/ml. LV: 15.625 - 1000 pg/ml. RG: 3.906 - 500 pg/ml. ON: 3.906 - 500 pg/ml. |
| Precision | Intra Assay Precision: Three human samples were diluted with Assay Buffer and run in replicates of 20 in an assay. The mean and precision of the calculated Prostaglandin E2 concentrations were: Sample 1- 11.7 pg/mL, 12.3% CV Sample 2- 98.6 pg/mL, 6.3% CV Sample 3- 131.2 pg/mL, 4.9% CV Inter Assay Precision: Three human samples were diluted with Assay Buffer and run in duplicates in seventeen assays run over multiple days by four operators. The mean and precision of the calculated Prostaglandin E2 concentrations were: Sample 1- 12.3 pg/mL, 8.8% CV Sample 2- 100.5 pg/mL, 8.1% CV Sample 3- 134.7 pg/mL, 9.8% CV |
| Number of Samples | 40 samples in duplicate |
| Other Resources | Kit Booklet , MSDS , Saliva Sample Handling Instructions , Eicosanoid Sample Extraction Protocol |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC | ||||||||||||||||||||||||||||||
| Shipping Temperature | Blue Ice | ||||||||||||||||||||||||||||||
| Product Type | EIA Kits | ||||||||||||||||||||||||||||||
| Assay Overview | The Prostaglandin E2 (PGE2) EIA kit is designed to quantitatively measure PGE2 present in serum, plasma, urine, saliva, cells, tissue, and tissue culture media samples. This EIA kit allows for the widest variations in sample size, sensitivity and assay timing of any PGE2 kit. The protocol variations are outlined below: The Regular Format, which uses 50 µL of sample or standard, is shown on pages 10-11, gives results in 2.5 hours. The Low Sample Volume Format, which uses 25 µL of sample or standard, is outlined on page 12-13, also gives results in 2.5 hours, but uses lower sample volumes. The High Sensitivity Format, which uses 100 µL of sample or standard, is shown on page 14-15, also gives results in 2.5 hours, but is the highest sensitivity kit of any type available. The Overnight Format, which uses 50 µL of sample or standard, is shown on page 16-17, and uses a primary overnight incubation at 4°C to fit your work flow. This format has similar sensitivity to the Regular Format outlined above. | ||||||||||||||||||||||||||||||
| Kit Overview |
|
||||||||||||||||||||||||||||||
| Cite This Product | Prostaglandin E2 EIA Kit (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SKT-207) |
Biological Description
| Alternative Names | (5Z,11α,13E,15S)-7-[3-hydroxy-2-(3-hydroxyoct-1-enyl)- 5-oxo-cyclopentyl] hept-5-enoic acid EIA Kit |
| Research Areas | Cancer, Cardiovascular System, Cell Signaling |
| Scientific Background | Eicosanoid signal transduction pathways are highly conserved and are involved in a number of physiological processes. Prostaglandins are synthesized from arachidonic acid by cyclooxygenase (COX)-1 or -2, which convert the acid into PGH2. This is further processed by cytosolic or microsomal prostaglandin synthases to become PGE2 or one of several other prostanoids (1-3). Prostacyclin is the major cyclooxygenase product in blood vessel walls and it is present in inflammatory fluids in similar concentrations to PGE2. Prostacyclin is a potent vasodilator and is more potent than PGE2 in producing hyperalgesia (4). PGE2 is produced by a wide variety of tissues (5-12) and in several pathological conditions, including inflammation, arthritis, fever, tissue injury, endometriosis, and a variety of cancers (5,6). Other biological actions of PGE2 include vasodilation, modulation of sleep/wake cycles, and facilitation of human immunodeficiency virus replication. It elevates cAMP levels, stimulates bone resorption, and has thermoregulatory effects. It has been shown to be a regulator of sodium excretion and renal hemodynamics (7-12). |
| References |
1. Moncada, S., Ferriera, SH. & Vane, JR. (1979). Handbook of Experimental Pharmacology,50/II. Pp. 588-616. Vane, J.R. & Ferreira, S.H. Berlin,New York: Springer. 2. Vane, JR. (1971). Nature,231, 232-235.3. 3. Willis, AL. (1969). In Prostaglandins, Peptides and Amines.Pp. 31-38. Ed. Mantegazza, P. & Horton, E.W.London: Academic Press. 4. Higgs, GA., Cardinal, DC., Moncada, S. & Vane, JR. (1979). Microvascular Res., 18,245-254. 5. Kargman, S. et al. (1996) Biochem Pharmacol. 52(7):1113-25 6. Thun MJ, Namboodiri MM, Heath CW Jr. New Engl. J. Med. (1991) 325: 1593–6. 7. Richardson PD and Withrington PG. Brit. J. Pharmacol. (1976) 57: 581-588. 8. O. Hayaishi. J. Biol. Chem. (1988) 263: 14593-14596. 9. S. Kuno, et al. Proc. Natl. Acad. Sci., USA, (1986) 83: 3487-3490. 10. D.L. Bareis, et al. Proc. Natl. Acad. Sci., USA, (1983) 80: 2514-2518. 11. L.G. Raisz, et al. Nature. (1977) 267: 532-534. 12. C.R. Long, Kinoshita Y, Knox FG. Prostaglandins. (1990) 40: 591-601. |
Product Images
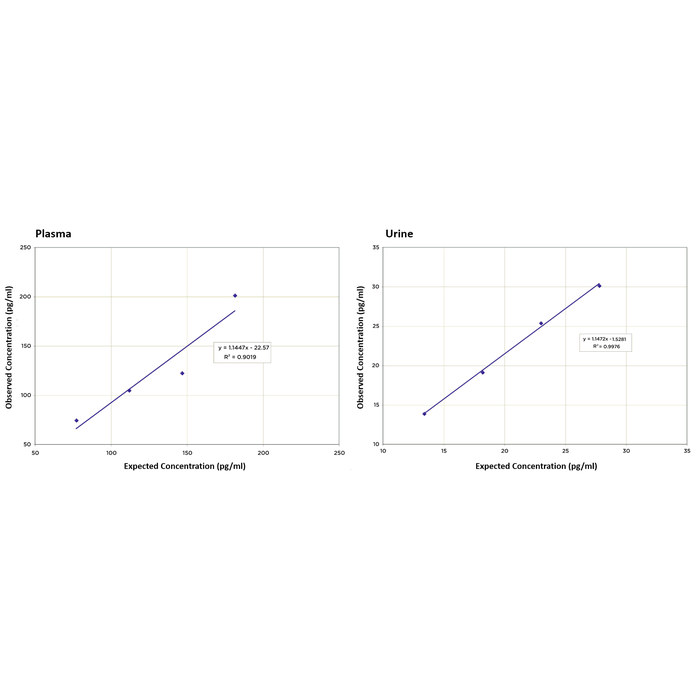
Linearity was determined in human plasma and urine samples by taking two diluted samples with known PGE2 concentrations. A plasma sample with a high PGE2 concentration of 216.4 pg/mL was mixed with one with a lower value of 42.5 pg/mL. A urine sample with a high PGE2 concentration of 32.6 pg/mL was mixed with one with a lower value of 8.6 pg/mL. They were mixed in the ratios. The measured concentrations were compared to the expected values based on the ratios used.




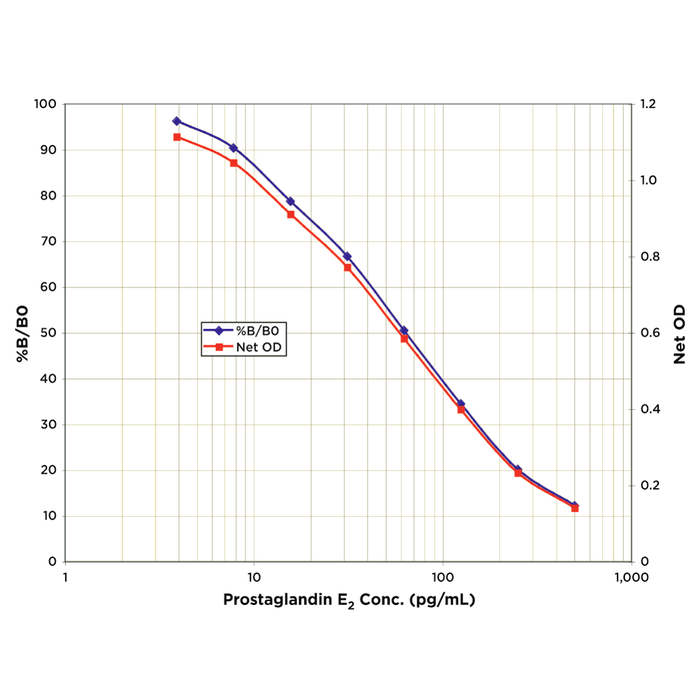
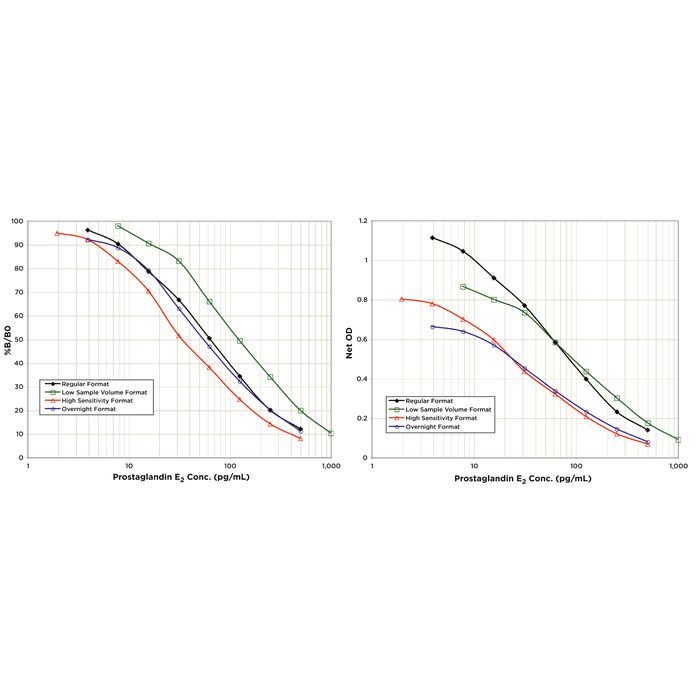
Reviews
There are no reviews yet.