Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Cynomolgus Recombinant Alpha Synuclein Pre-formed Fibrils: Biotinylated (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-528) |
| Certificate of Analysis | Protein certified > 95% pure via SDS-PAGE and nanodrop analysis. Low endotoxin (< 5 EU/mL) at 2 mg/mL. |
| Other Relevant Information | For corresponding monomer see catalog# SPR-527 (unbiotinylated version not available yet but coming soon) |
Biological Description
| Alternative Names | Alpha-synuclein, α-synuclein, SNCA, Synelfin, NACP, Non-Aβ component of Alzheimer's disease amyloid protein, Non-A beta component precursor, NACP140, PARK1 protein, PARK4 protein, Synuclein alpha, ASYN, Alpha-syn, Macaca fascicularis, UniProt: A0A2K5XN13 |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Cellular Localization | Protein certified > 95% pure via SDS-PAGE and nanodrop analysis. Low endotoxin (< 5 EU/mL) at 2 & 5 mg/mL. |
| Swiss Prot | P61142 |
| Scientific Background |
Alpha‑synuclein is a 140‑amino‑acid neuronal protein encoded by the SNCA gene and is highly enriched at presynaptic terminals, where it regulates synaptic vesicle trafficking, neurotransmitter release, and membrane interactions. In neurodegenerative disorders such as Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy, misfolded alpha‑synuclein adopts aggregation‑prone conformations that progress from soluble monomers to toxic oligomers and fibrils, ultimately forming Lewy body pathology and driving cellular dysfunction and neurodegeneration. Biotinylated alpha‑synuclein pre‑formed fibrils (PFFs) are powerful molecular tools that enable precise tracking, quantification, and manipulation of pathogenic fibrillar species. Because PFFs replicate the structural and biochemical characteristics of disease‑associated fibrils—including amyloid‑like β‑sheet conformation and seeding capacity—they provide a controlled system for inducing endogenous alpha‑synuclein aggregation in cellular and in vivo models. When biotinylated, these fibrils can be selectively captured, imaged, or enriched, allowing researchers to dissect early aggregation events, map protein interactomes, and monitor fibril propagation with exceptional sensitivity. Biotinylated PFFs also support mechanistic studies on prion‑like spread, synaptic dysfunction, mitochondrial impairment, and neuroinflammatory signaling, all of which are central to synucleinopathy progression. By enabling reproducible seeding assays and high‑resolution interrogation of fibril–cell interactions, they accelerate therapeutic discovery efforts targeting fibril formation, uptake, clearance, and downstream pathological cascades. As a result, biotinylated alpha‑synuclein PFFs have become essential reagents for advancing translational neurodegeneration research and for developing next‑generation strategies to halt or reverse protein‑misfolding diseases. A 15 amino acid tag on the C-terminal tail of alpha synuclein facilitates site-specific covalent biotinylation. Biotinylation of purified alpha synuclein allows for many biological applications, such as monitoring and detection using streptavidin-based conjugates. Stressmarq’s pre-formed fibrils are generated from biotinylated alpha synuclein monomers. |
| References |
1.,Fairhead & Howarth. 2015. Site-specific biotinylation of purified proteins using BirA. Methods in Molecular Biology. DOI: 1007/978-1-4939-2272-7_12 2.,Hallacli et al. 2022. The Parkinson’s disease protein alpha synuclein is a modulator of Processing-bodies and mRNA stability. Cell. DOI: 10.1016/j.cell.2022.05.008 3.,Li et al. 2019. Naturally occurring antibodies isolated from PD patients inhibit synuclein seeding in vitro and recognize Lewy pathology. Acta neuropathologica. DOI: 10.1007/s00401-019-01974-5. |
Product Images
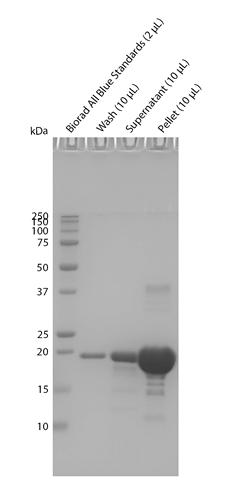
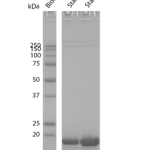
Sedimentation assay on Cynomolgus Alpha Synuclein Pre-Formed Fibrils: Biotinylated (C-Terminus). Samples were spun down at 15,000 x g, washed, and then spun down again. Fibril samples are prepared in denaturing conditions prior to running on the gel. SDS-PAGE analysis on a 12% Bis-Tris gel shows that the majority of the fibril is insoluble.
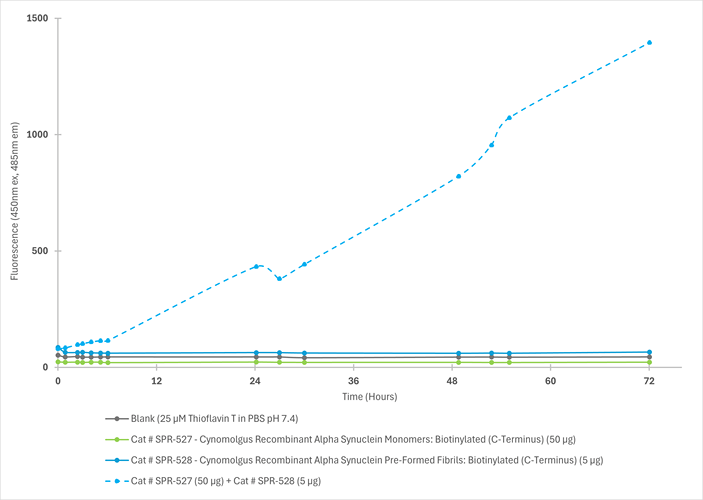
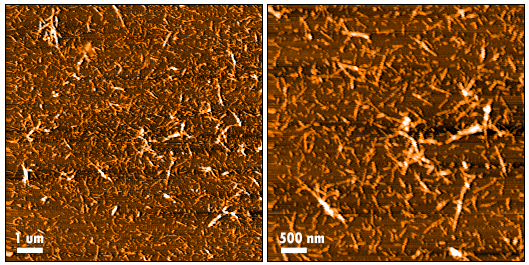
In vitro seeding activity of Cynomolgus Alpha Synuclein Pre-Formed Fibrils: Biotinylated (C-Terminus) in a Thioflavin T (ThT) assay. Cynomolgus Alpha Synuclein Pre-Formed Fibrils: Biotinylated (C-Terminus) (SPR-528) seed fibril formation of Cynomolgus Alpha Synuclein Monomers: C-Term Biotinylated (SPR-527) over 72 hours. Reactions (100uL) shaken at 600 rpm in Greiner-Bio 96 Well Non-Binding Cell Culture Microplates, Black (Greiner-Bio Catalog #655900) at 37℃ in the presence of 25 uM ThT and read with an XPS Microplate Reader set at 450nmex/485nmem.
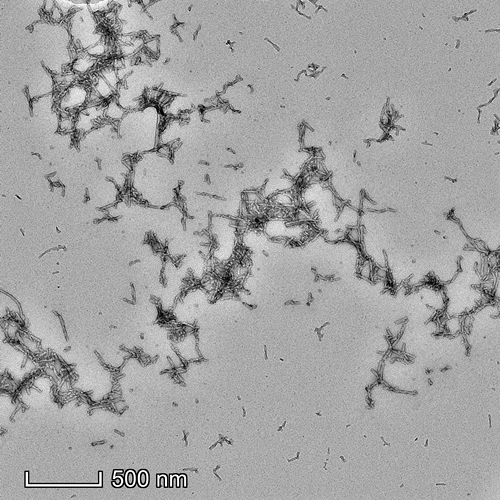
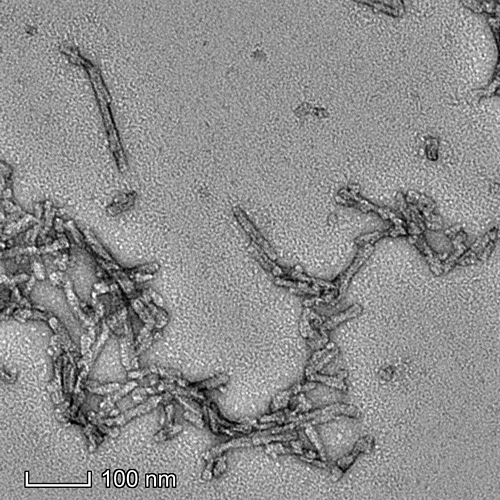
Representative TEM image of Cynomolgus Recombinant Alpha Synuclein Pre-Formed Fibrils: Biotinylated (C-Terminus), 500nm scale. Negative stain transmission electron microscopy images of SPR-528 acquired at 80 Kv on carbon coated 400 mesh copper grids using phosphotungstic acid and uranyl acetate stain.
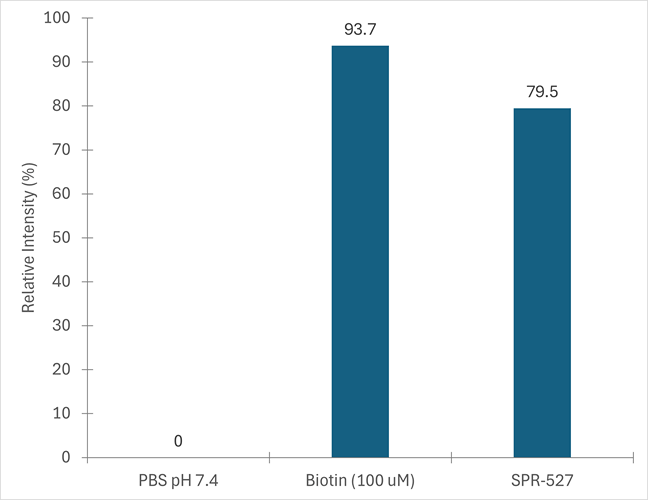
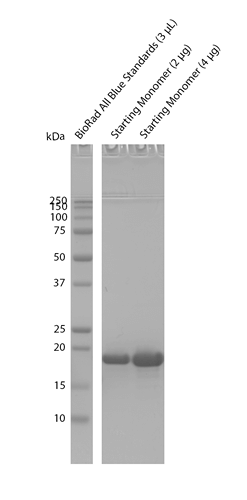
Biotinylation assay on purified Cynomolgus Alpha Synuclein Monomers: Biotinylated (C-Terminus). The assay is performed on 123 uM of monomers post-biotinylation (SPR-527), which is the starting material used to generate the Pre-Formed Fibrils (SPR-528), with 100 uM of biotin as a positive control & PBS pH 7.4 as a negative control. Absorbance is measured at 500nm, and results are converted to % of biotinylation.





















Reviews
There are no reviews yet.