Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Alpha Synuclein pSer129 Monomers (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-520) |
| Certificate of Analysis | Protein certified >95% pure using SDS-PAGE analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For corresponding PFFs, see Catalog# SPR-521. The unphosphorylated construct is Catalog# SPR-321. |
Biological Description
| Alternative Names | Alpha-synuclein, Alpha synuclein, Non-A beta component of AD amyloid, Non-A4 component of amyloid precursor, NACP, SNCA, PARK1, SYN, PD1, PARK4, Synuclein Alpha, Alpha Synuclein pSer129, Asyn |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Swiss Prot | P37840 |
| Scientific Background |
Alpha-synuclein (α-synuclein), encoded by the SNCA gene, is a neuronal protein involved in synaptic vesicle trafficking, neurotransmitter release, and SNARE-complex assembly. In neurodegenerative diseases such as Parkinson’s disease, α-synuclein undergoes pathological modifications, with phosphorylation at serine 129 (pSer129) being the most prominent post-translational change observed in aggregated forms within Lewy bodies. Phosphorylated α-synuclein at Ser129 is considered a biomarker of disease-associated aggregation and neurotoxicity. Monomeric pSer129 α-synuclein allows researchers to isolate and study the functional consequences of this specific modification. These monomers exhibit altered conformational dynamics, aggregation propensity, and cellular interactions compared to their non-phosphorylated counterparts. In neurodegenerative disease models, pSer129 monomers are used to investigate early misfolding events, membrane binding behavior, and the role of phosphorylation in promoting toxic oligomer formation. Their application supports mechanistic studies on synaptic dysfunction, mitochondrial impairment, and neuroinflammatory signaling pathways. Alpha-synuclein pSer129 monomers are also valuable in therapeutic screening platforms, enabling the evaluation of compounds that target phosphorylation-dependent aggregation or enhance clearance of modified α-synuclein. By replicating disease-relevant molecular features, these monomers accelerate translational research aimed at understanding and mitigating the progression of Parkinson’s disease and related synucleinopathies. StressMarq's Alpha Synuclein Ser129 Monomers are generated in-house and phosphorylation confirmed with our anti-ASYN pS129 monoclonal antibody (catalog# SMC-600). |
| References |
1. Okochi et al. 2000. Constitutive phosphorylation of the Parkinson’s Disease Associated a-Synuclein. The Journal of Biological Chemistry. DOI: 10.1074/jbc.275.1.390 2. Fujiwara et al. 2002. α-Synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biology. DOI: 10.1038/ncb748 3. Magalhaes and Lashuel. 2002. Opportunities and challenges of alpha-synuclein as a potential biomarker for Parkinson’s disease and other synucleinopathies. Npj Parkinsons Disease. DOI: 10.1038/s41531-022-00357-0 |
Product Images
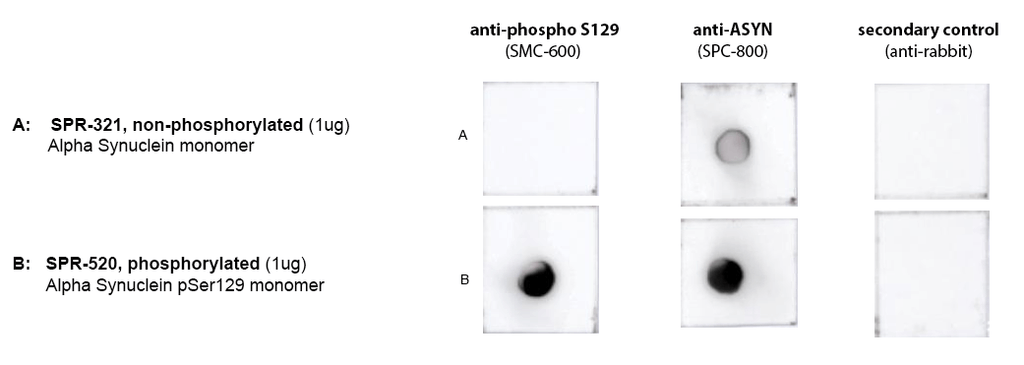
Dot Blot of purified Human Recombinant Alpha Synuclein pSer129 Monomers (SPR-520) using Stressmarq’s SMC-600 (anti-ASYN pS129) and SPC-800 (anti-ASYN) confirming phosphorylation in SPR-520, compared to SPR-321. Protein was blotted on nitrocellulose, incubated with 1:1000 primary antibodies and/or 1:4000 secondary antibodies. Secondary control is goat-anti rabbit:HRP. Exposed 1 second.

Modified/total phosphorylation PTM spectrum counts of 4 sites on human alpha synuclein pSer129 monomers (SPR-520) as determined by mass spectrometry (ScaffoldPTM, localization probability = 100% at both 0% and 95% min. localization). CNBr digestion was used to accurately determine the presence of phosphorylation at and around S129. Note that total counts may include longer peptides where phosphorylation may be more difficult to detect, and most phospho-modified sites that were detected are at S129.
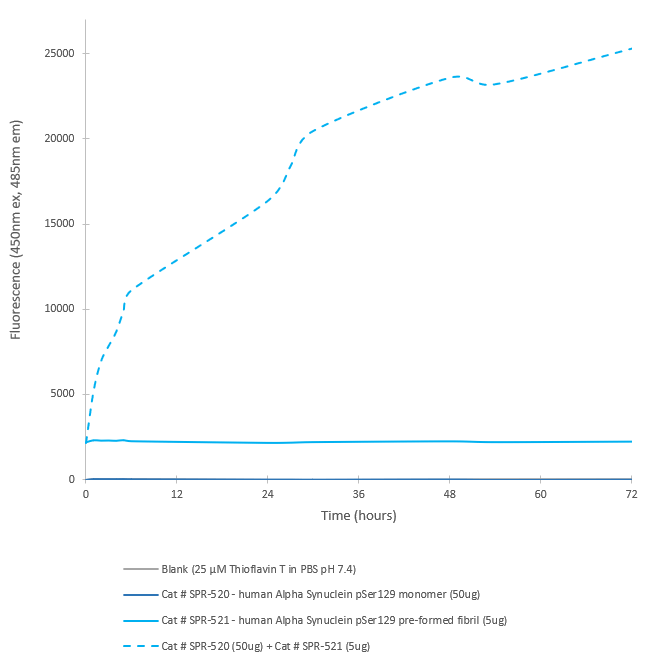
In vitro seeding activity of Alpha Synuclein pSer129 Monomers in ThT assay. Alpha Synuclein pSer129 Pre-Formed Fibrils (SPR-521) seed fibril formation of Alpha Synuclein pSer129 Monomers (SPR-520) over 72 hours. Reactions (100uL) shaken at 600 rpm in Greiner-Bio 96 Well Non-Binding Cell Culture Microplates, Black (Greiner-Bio Catalog #655900) at 37℃ in the presence of 25 uM ThT and read with an XPS Microplate Reader set at 450nmex/485nmem.





















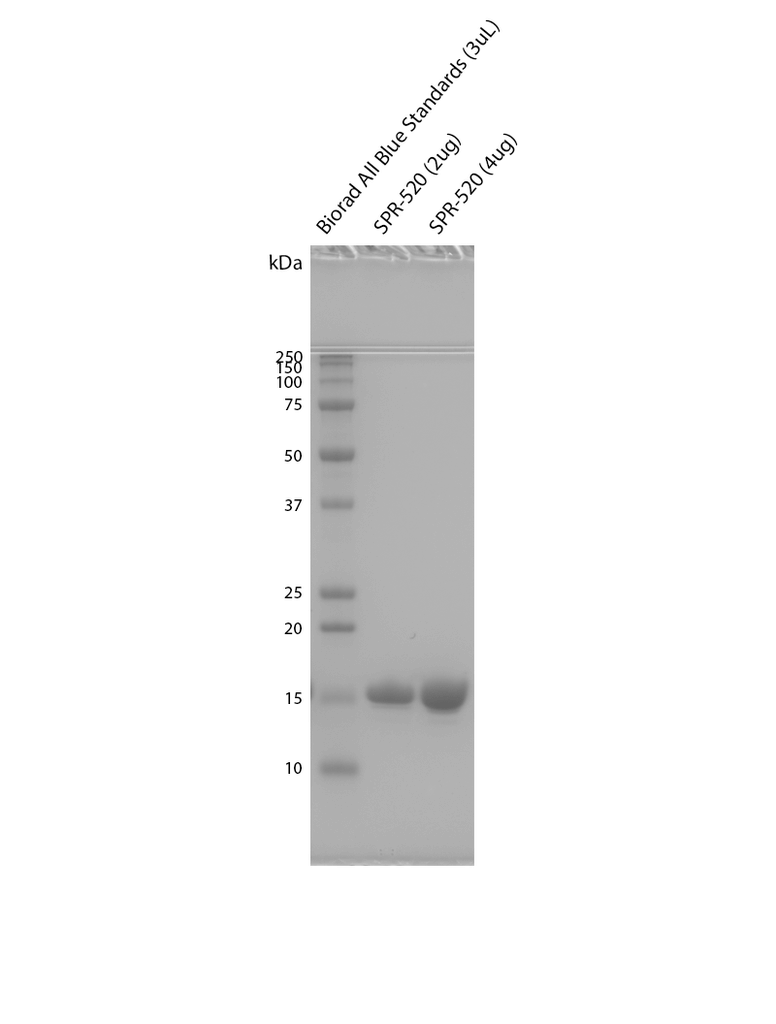
Reviews
There are no reviews yet.