| Product Name | Alpha Synuclein TNG (A53T, S87N, N103G) Mutant Pre-formed Fibrils | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description |
Human Recombinant Alpha Synuclein TNG (A53T, S87N, N103G) Mutant PFFs |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | WB, SDS PAGE, In vitro Assay | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentration | 2 mg/ml or 5 mg/ml | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conjugates |
No tag
StreptavidinProperties:
Biotin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nature | Recombinant | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Human | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Expression System | E. coli | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amino Acid Sequence | MDVFMKGLSKAKEGVVAAAEKTKQGVAEAAGKTKEGVLYVGSKTKEGVVHGVTTVAEKTKEQVTNVGGAVVTGVTAVAQKTVEGAGNIAAATGFVKKDQLGKGEEGAPQEGILEDMPVDPDNEAYEMPSEEGYQDYEPEA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purity | >95% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Resources | Sonication Protocol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Protein Length | Full length (1 - 140 aa) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Protein Size | ~14.46 kDa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Properties
| Storage Buffer | 1X PBS pH7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Alpha Synuclein TNG (A53T, S87N, N103G) Mutant Pre-formed Fibrils (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-504) |
| Certificate of Analysis | Protein certified >95% pure on SDS-PAGE & Nanodrop analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For best results, sonicate immediately prior to use. Refer to the Neurodegenerative Protein Handling Instructions on our website, or the product datasheet for further information. Monomer source is catalog# SPR-503. |
Biological Description
| Alternative Names | Alpha Synuclein TNG, Alpha-synuclein, Non-A beta component of AD amyloid, Non-A4 component of amyloid precursor, NACP, SNCA, PARK1, SYN, PD1, PARK4, Synuclein Alpha, Asyn, Alpha Synuclein PFFs |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Swiss Prot | P37840-1 |
| Scientific Background |
The TNG variant of alpha-synuclein—comprising A53T, S87N, and N103G mutations—represents a strategically engineered mutant designed to enhance aggregation propensity and mimic disease-relevant biochemical behavior. Human alpha synuclein TNG mutant (HuTNG) is a triple mutant containing Ala53 mutated to the equivalent mouse residue Thr53, Ser87 mutated to the equivalent mouse residue Asn87, and Asn103 mutated to the equivalent mouse residue Gly103, effectively making it a human-mouse chimeric protein. Despite sequence differences at only seven residues, or 5% of the total 140 amino acids, the aggregation rate of wild-type mouse α-syn (MsWT) is faster than wild-type human α-syn (HuWT) in vitro. In wild-type mouse models, MsWT fibrils are more efficient than HuWT fibrils at inducing endogenous mouse α-syn pathology (1). A53T or S87N substitutions in human α-syn substantially accelerate fibrilization rates in vitro (2,3). Chimeric HuTNG fibrils show enhanced induction of α-syn pathology greater than both HuWT and MsWT fibrils after single unilateral injection into the dorsal striatum in mice (4). Therefore, HuTNG is a good construct for inducing robust endogenous α-syn seeding and pathology in wild-type mice. TNG mutant monomers serve as a powerful tool for modeling early-stage α-synuclein pathology. They enable precise investigation of misfolding dynamics, oligomer formation, and cellular toxicity in vitro and in vivo. Their use facilitates the study of molecular mechanisms underlying synaptic dysfunction, mitochondrial impairment, and neuroinflammation—hallmarks of neurodegenerative progression. By replicating key aspects of α-synuclein-driven disease, TNG mutant monomers support high-throughput screening of therapeutic candidates aimed at stabilizing native protein structure, preventing aggregation, and restoring neuronal function. Their application accelerates translational research targeting Parkinson’s disease and related disorders. |
| References |
1. Masuda-Suzukake et al. 2013. Prion-like Spreading of Pathological α-synuclein in Brain. Brain. https://doi.org/10.1093/brain/awt037 2. Kang, K. et al. 2011. The A53T Mutation is Key in Defining the Differences in the Aggregation Kinetics of Human and Mouse α-synuclein. JACS. https://doi.org/10.1021/ja203979j 3. Ohgita, T. et al. 2023. Intramolecular Interaction Kinetically Regulates Fibril Formation by Human and Mouse Alpha-Synuclein. Sci Rep https://doi.org/10.1038/s41598-023-38070-4 4. Luk, K., C. et al. 2016. Molecular and Biological Compatibility with Host Alpha-Synuclein Influences Fibril Pathogenicity. Cell Rep. https://doi.org/10.1016/j.celrep.2016.08.053 |
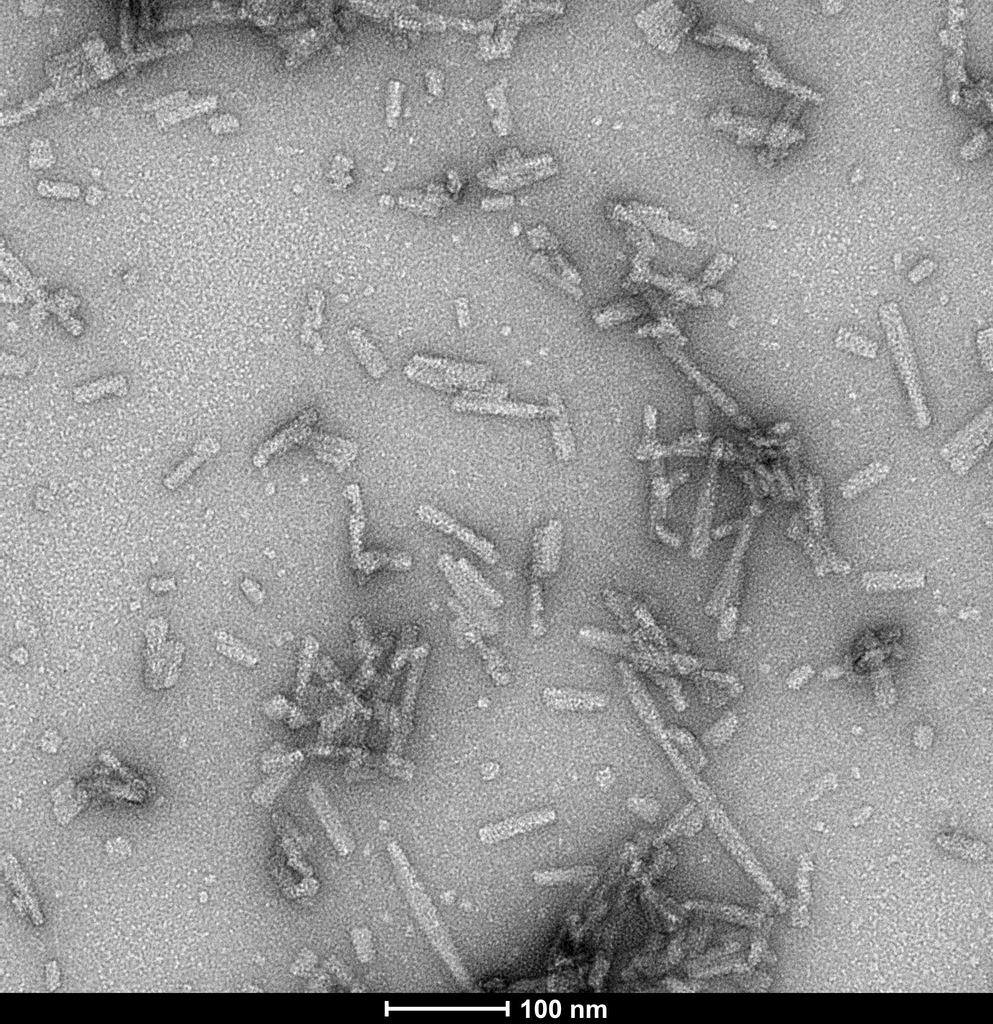
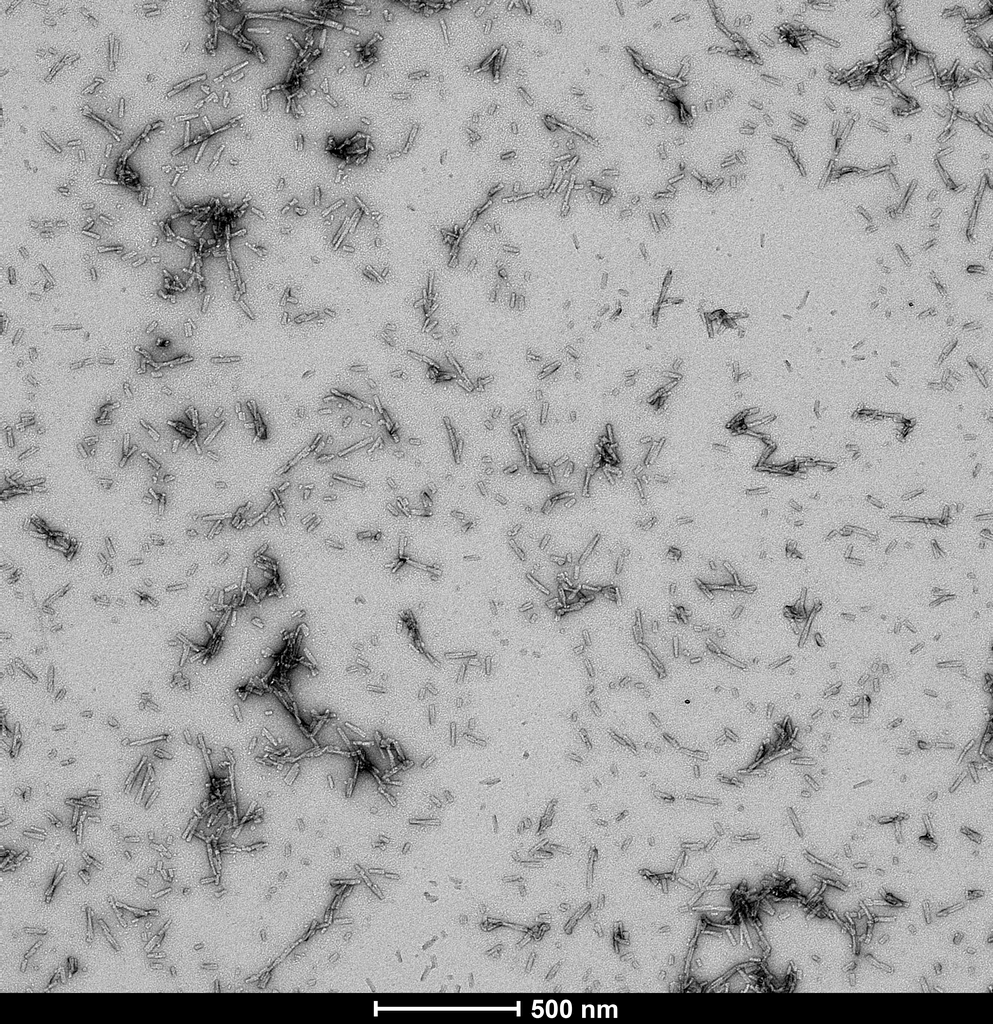
Product Images
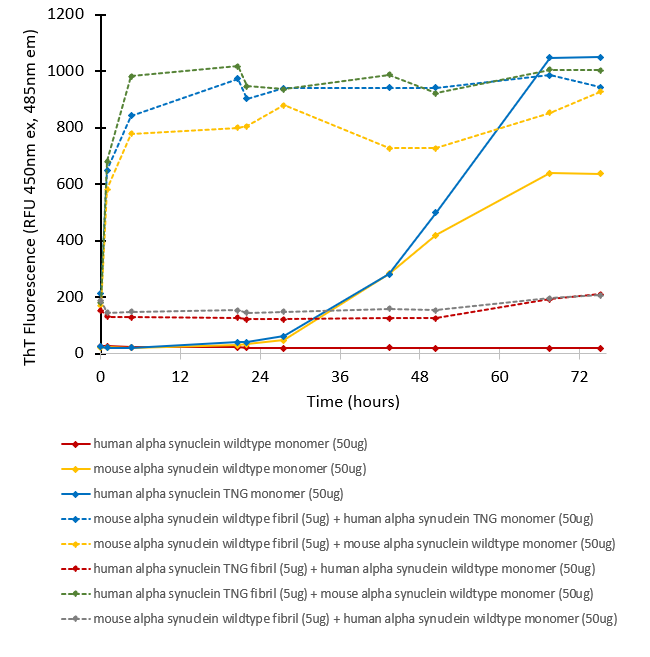
Fibril formation and seeding activity of human alpha synuclein TNG mutant measured by ThT in vitro. Human TNG mutant monomers (SPR-503) self-aggregate faster than human wild-type monomers. Human TNG mutant pre-formed fibrils (SPR-504) rapidly seed mouse wild-type monomers, but not human wild-type monomers, similar to the seeding pattern observed for mouse wild-type pre-formed fibrils.

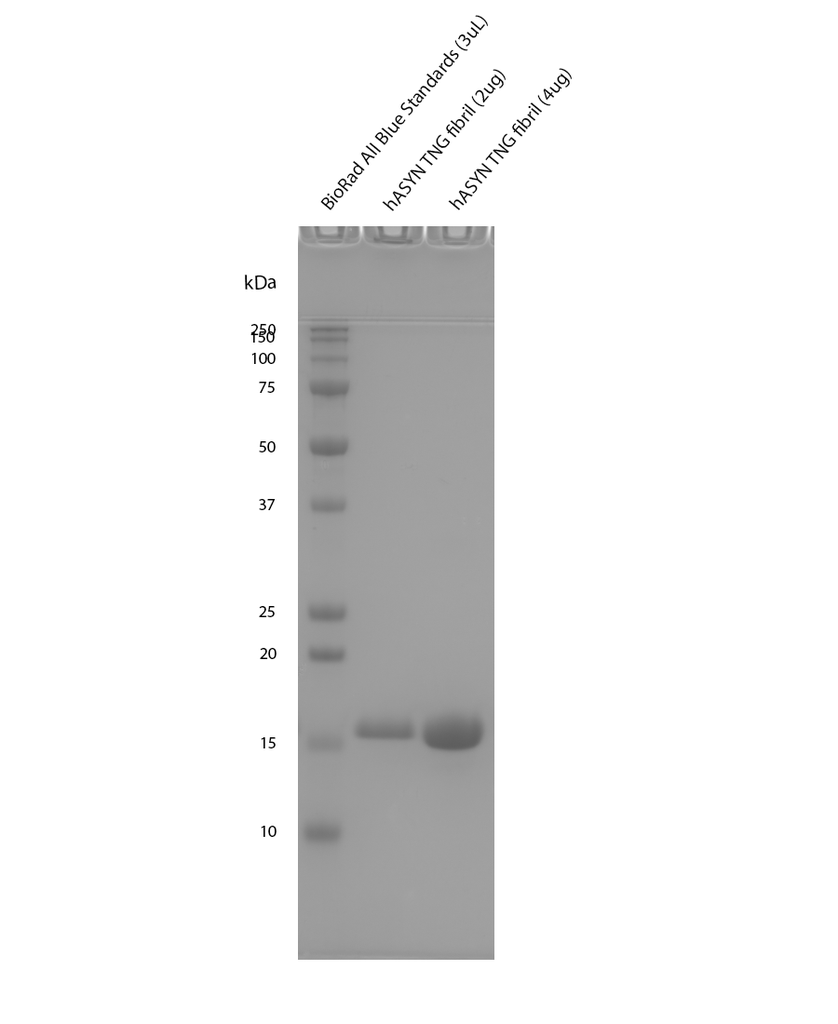
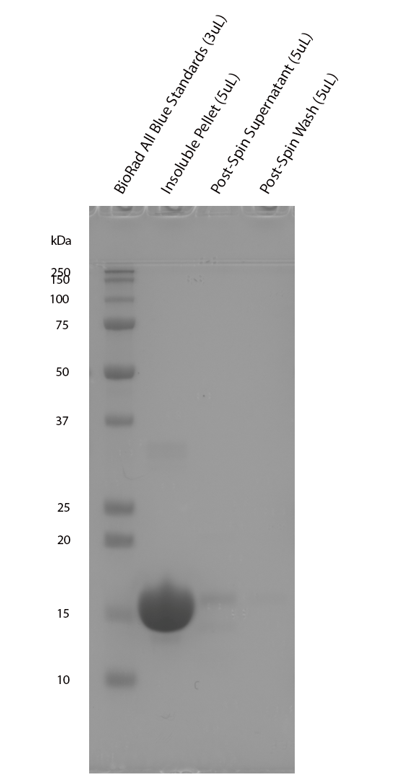





















Reviews
There are no reviews yet.