Properties
| Storage Buffer | PB pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant SOD1 Monomers (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-435) |
| Certificate of Analysis | Certified >95% pure using SDS-PAGE analysis. |
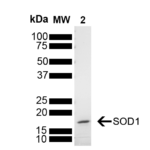
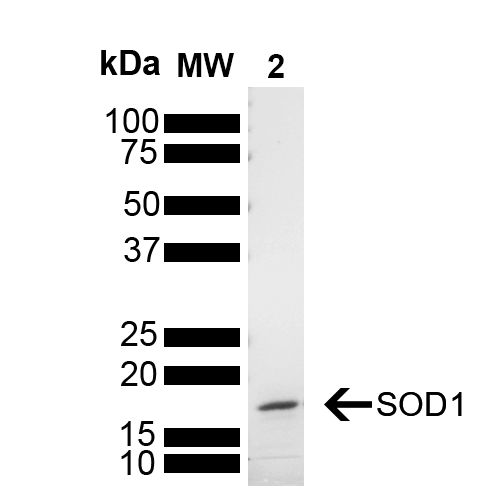
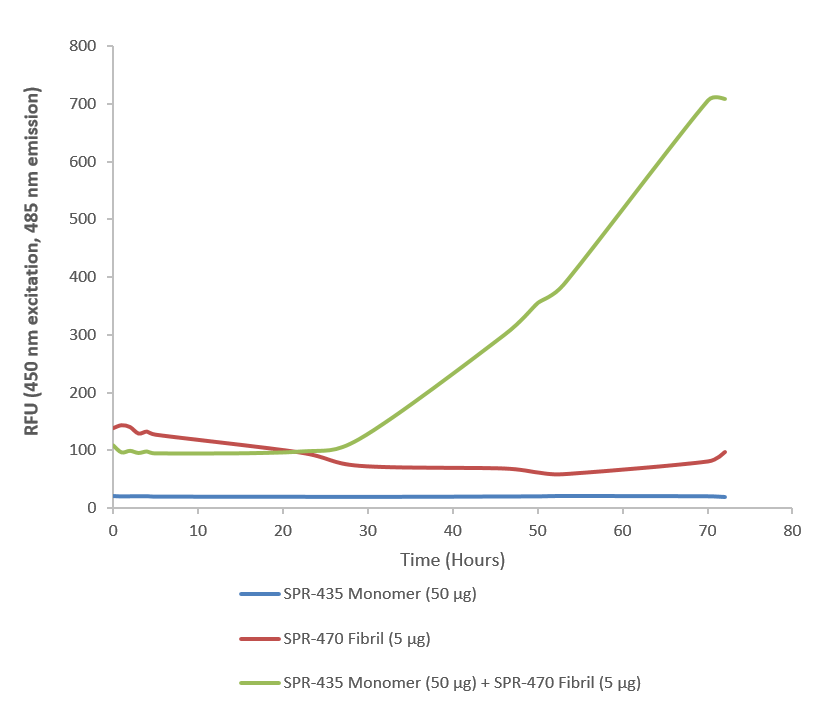
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-470 |
Biological Description
| Alternative Names | Superoxide dismutase 1, SOD1, ALS1, IPOA, Cu/Zn superoxide dismutase, Superoxide dismutase [Cu-Zn], hSOD1, SODC, Cytosolic superoxide dismutase, Erythrocuprein, Hemocuprein, Cytocuprein, Indophenoloxidase A |
| Research Areas | ALS Disease, Cancer, Cell Signaling, Chaperone Proteins, Neurodegeneration, Neuroscience, Oxidative Stress, Protein Trafficking |
| Cellular Localization | Cytoplasm, Mitochondrion, Nucleus |
| Accession Number | NP_000445.1 |
| Gene ID | 6647 |
| Swiss Prot | P00441 |
| Scientific Background |
Superoxide dismutase 1 (SOD1), encoded by the SOD1 gene, is a critical antioxidant enzyme that normally functions as a stable homodimer to neutralize superoxide radicals. However, in neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), mutations in SOD1 destabilize its dimeric structure, leading to the formation of toxic monomeric species. SOD1 monomers are increasingly recognized as central players in ALS pathogenesis. These misfolded monomers exhibit a high propensity for aggregation, triggering a cascade of cellular dysfunctions including mitochondrial damage, impaired axonal transport, and activation of apoptotic pathways. Their accumulation in motor neurons contributes to progressive neurodegeneration and loss of motor function. Advanced structural studies have revealed that mutant SOD1 monomers undergo conformational changes that expose hydrophobic regions, promoting aggregation and toxicity. These insights have catalyzed the development of therapeutic strategies aimed at stabilizing SOD1 dimers or preventing monomer formation. Beyond ALS, emerging evidence suggests that SOD1 monomers may influence oxidative stress responses in other neurodegenerative conditions, positioning them as potential biomarkers and therapeutic targets. Understanding their molecular behavior is essential for designing precision interventions that mitigate protein misfolding and neuronal damage. In summary, SOD1 monomers represent a critical nexus in neurodegenerative disease research, offering new opportunities for diagnostics and targeted therapies that address the root causes of neuronal vulnerability. |
| References |
1. Adachi T., et al. (1992). Clin. Chim. Acta. 212: 89-102. 2. Barrister J.V., et al. (1987). Crit. Rev. Biochem. 22:111-180. 3. Furukawa Y., O'Halloran T. (2006). Antioxidants & Redo Signaling. Vol 8, No 5,6. 4. Gao B., et al. (2003). Am J Physiol Lung Cell Mol Physiol 284: L917-L925. 5. Hassan H.M. (1988). Free Radical Biol. Med. 5: 377-385. 6. Kurobe N., et al. (1990) Biomedical Research. 11: 187-194 7. Wispe J.R., et al. (1989) BBA. 994: 30-36. 8. Xiao-Hong Liu., et al. (1993) Brain Research. 625: 29-37. 9. Furukawa Y., et al. (2013) FEBS 587(16): 2500-2505. |






















Reviews
There are no reviews yet.