Properties
| Storage Buffer | 10mM HEPES pH 7.4, 100mM NaCl |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Tau (K18) P301L Mutant Pre-formed Fibrils (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-523) |
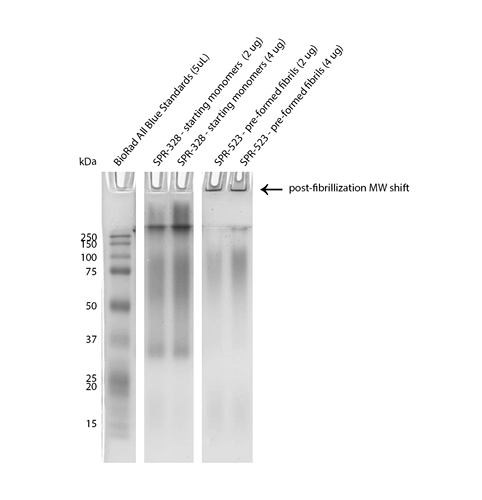
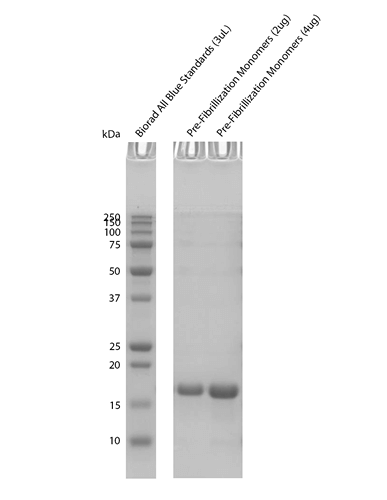
| Certificate of Analysis | Protein certified > 95% pure via SDS-PAGE and A260/A280 ratio |
| Other Relevant Information | Monomer source is SPR-328 |
Biological Description
| Alternative Names | Tau K18 P301L, K18-P301L Tau, Tau 4R repeat domain P301L, Tau MTBR P301L, Tau microtubule-binding region P301L, Tau repeat domain P301L (Tau-RD P301L), Tau K18 mutant P301L, Recombinant Tau K18 P301L fragment, Tau 4-repeat construct P301L, Tau RD(P301L), Tau K18 familial FTDP-17 mutant, Tau PFFs |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Tangles & Tau |
| Swiss Prot | P10636 |
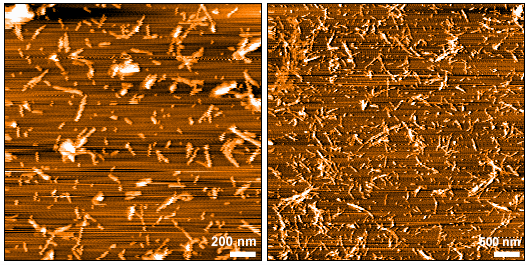
| Scientific Background | Tauopathies are a class of neurodegenerative diseases characterized by the pathological aggregation of tau protein. The K18 fragment, comprising the microtubule-binding repeat domains of tau, is frequently used in experimental models due to its propensity to form fibrillar aggregates. The P301L mutation, located within the repeat domain, has been demonstrated to increase the protein's propensity to aggregate and seed, making it a key variant in modeling tau pathology. StressMarq's Tau (K18) P301L Mutant Pre-formed Fibrils are fibrillized without heparin and have been demonstrated to be neurotoxic to primary mouse cortical neurons. |
| References |
1.,Peeraer, E., Bottelbergs, A., Van Kolen, K., Stancu, I. C., Vasconcelos, B., Mahieu, M., Duytschaever, H., Ver Donck, L., Torremans, A., Sluydts, E., Van Acker, N., Kemp, J. A., Mercken, M., Brunden, K. R., Trojanowski, J. Q., Dewachter, I., Lee, V. M., & Moechars, D. (2015). Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiology of disease, 73, 83–95. https://doi.org/10.1016/j.nbd.2014.08.032 2.,Croft, C. L., Goodwin, M. S., Ryu, D. H., Lessard, C. B., Tejeda, G., Marrero, M., Vause, A. R., Paterno, G., Cruz, P. E., Lewis, J., Giasson, B. I., & Golde, T. E. (2021). Photodynamic studies reveal rapid formation and appreciable turnover of tau inclusions. Acta Neuropathologica, 141, 359–381. https://doi.org/10.1007/s00401-021-02264-9 3.,Zhang, X., Wang, J., Zhang, Z., & Ye, K. (2024). Tau in neurodegenerative diseases: molecular mechanisms, biomarkers, and therapeutic strategies. Translational Neurodegeneration, 13(40). https://doi.org/10.1186/s40035-024-00429-6 |
Product Images
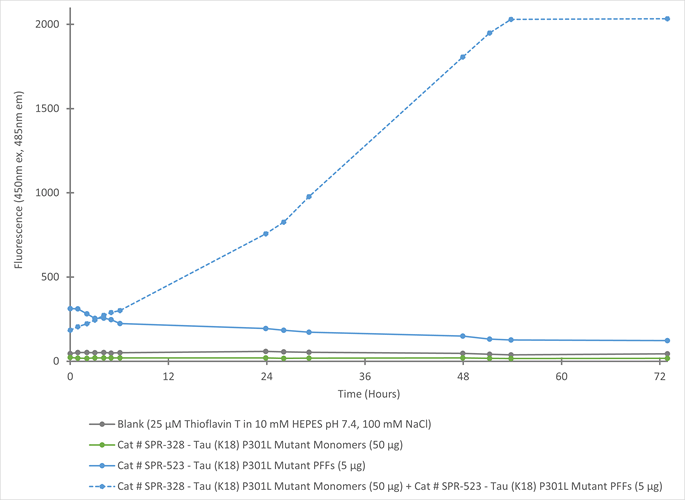
In vitro seeding activity of Tau (K18) P301L Mutant Pre-Formed Fibrils in a ThT assay. Tau (K18) P301L Mutant Pre-Formed Fibrils (SPR-523) seed fibril formation of Tau (K18) P301L Mutant Pre-Formed Fibrils (SPR-328) over 72 hours. Reactions (100uL) are shaken at 600 rpm in Greiner-Bio 96 Well Non-Binding Cell Culture Microplates, Black (Greiner-Bio Catalog #655900) at 37C in the presence of 25 uM ThT and read with an XPS Microplate Reader set at 450nm ex/485nm em.
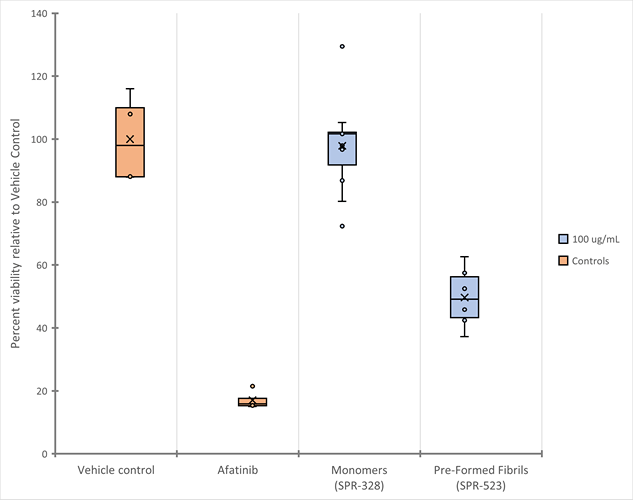
Tau (K18) P301L Mutant Pre-Formed Fibrils (average = 49.7%, SD = 9.6, n = 6) demonstrate increased toxicity to primary mouse cortical neurons compared to the Tau (K18) P301L Mutant Monomers (average = 97.9%, SD = 14.9, n = 11). The protein constructs and a control (10 µM Afatinib) were added to the neuron cultures at 12 DIV and toxicity assessed via a CCK-8 assay at 15 DIV.























Reviews
There are no reviews yet.