Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Transthyretin (TTR) L55P Variant Monomers (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-451) |
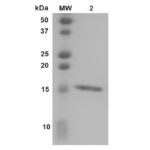
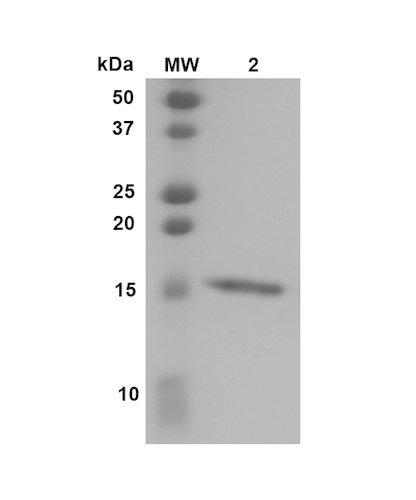
| Certificate of Analysis | Certified >95% pure using SDS-PAGE analysis. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-464 |
Biological Description
| Alternative Names | Transthyretin L55P, TTR L55P, ATTR, Amyloid polyneuropathy, Amyloidosis I, Carpal tunnel syndrome 1, CTS, CTS1, HEL111, HsT2651, PALB, Prealbumin, Prealbumin amyloidosis type I, Prealbumin Thyroxine-binding, TBPA, Thyroxine binding prealbumin, TTHY_HUMAN, TTR |
| Research Areas | ALS Disease, Alzheimer's Disease, Blood, Cardiovascular System, Cell Signaling, Lipid and lipoprotein Metabolism, Metabolism, Neurodegeneration, Neuroscience, Parkinson's Disease, Tangles & Tau |
| Cellular Localization | Cytoplasm, Extracellular exosome, Extracellular Region, Lysosome |
| Accession Number | NP_000362.1 |
| Gene ID | 7276 |
| Swiss Prot | P02766 |
| Scientific Background | Transthyretin is a transport protein in the serum and cerebospinal fluid that carried the thyroid hormone Thyroxine and retinol-binding protein bound to retinol. TTR misfolding and aggregation is known to be associated with the amyloiddiseases SSA, FAP and FAC (1-5). TTR is also thought to have beneficial side effects, such as binding to beta-amyloid protein, preventing beta-amyloid from accumulating into the plaques associated with Alzheimer's Disease (6). The L55P variant TTR is distinct from the other variants in that the L55P tetramer can dissociate to the monomeric amyloidogenic intermediate and form fibril precursors (7). |
| References |
1. Zeldenrust S.R., Benson M.D. (2010). Wiley. pp. 795–815. 2. Westermark P., Sletten K., Johansson B., Cornwell G.G. (1990). Proc. Natl. Acad. Sci. U.S.A. 87(7): 2843–5. 3. Andrade C. (1952). Brain. 75(3): 408–27. 4. Coelho T. (1996). Curr. Opin. Neurol. 9(5): 355–9. 5. Jacobson D.R, et. al. (1997). N. Engl. J. Med. 336(7): 466–73. 6. Li X. (2011). Mol Neurodegener. 6(1):79. 7. Lashuel H.A., Wurth C., Woo L., and Kelly J.W. (1999) Biochem. 38(41): 13560-13573. |





















Reviews
There are no reviews yet.