Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Alpha Synuclein A53T Mutant Monomers (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-325) |
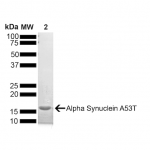
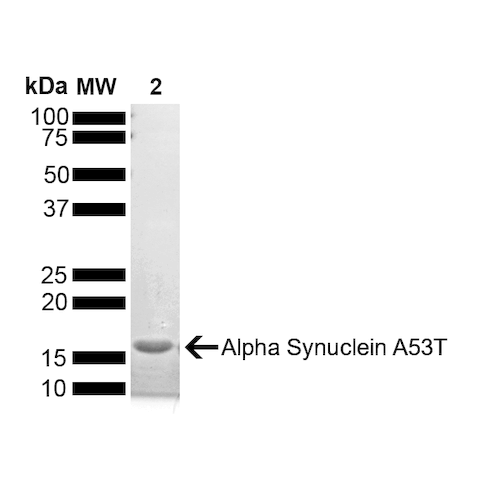
| Certificate of Analysis | Certified >95% pure using SDS-PAGE analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-325 |
Biological Description
| Alternative Names | Alpha-synuclein A53T, A53T mutant alpha-synuclein, A53T mutated SNCA, Ala53Thr mutant alpha-synuclein, Asyn, SNCA, NACP, PARK1, PARK4, PD1, Synuclein alpha, Non-A beta component of AD amyloid, Non-A4 component of amyloid precursor, Synuclein Alpha-140, SYN, Parkinson's disease familial 1 Protein |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Cellular Localization | Cytoplasm, Membrane, Nucleus |
| Accession Number | NP_000336.1 |
| Gene ID | 6622 |
| Swiss Prot | P37840 |
| Scientific Background |
Alpha-synuclein (α-syn), encoded by the SNCA gene, is a neuronal protein predominantly localized at presynaptic terminals, where it regulates synaptic vesicle trafficking, neurotransmitter release, and SNARE-complex assembly. In its monomeric form, α-syn plays a crucial role in maintaining synaptic integrity and cognitive function. The A53T mutation in α-syn, characterized by a substitution of alanine with threonine at position 53, is linked to familial early-onset Parkinson’s disease. This missense mutation alters the biophysical properties of α-syn monomers, increasing their propensity to misfold and aggregate into toxic oligomers and fibrils. These aggregates disrupt neuronal homeostasis, impair mitochondrial function, and trigger neuroinflammatory responses, contributing to progressive neurodegeneration. Studies have shown that A53T mutant monomers exhibit enhanced fibrillization kinetics and altered interactions with cellular components, including microtubules and SNARE proteins. Additionally, the mutation compromises α-syn’s ability to regulate exocytotic fusion pore dynamics, a function critical for neurotransmitter release. The A53T variant also influences proteolytic processing, generating truncated peptides that exacerbate neurotoxicity without forming classical Lewy body aggregates. Understanding the molecular mechanisms by which A53T mutant monomers drive pathology is essential for developing targeted therapies. Interventions aimed at stabilizing monomeric α-syn, preventing aggregation, or modulating its interactions with cellular machinery offer promising avenues for mitigating disease progression in Parkinson’s and related synucleinopathies. |
| References |
1. “Genetics Home Reference: SNCA”. US National Library of Medicine. (2013). 2. Zhang L., et al. (2008) Brain Res. 1244: 40-52. 3. Alim M.A., et al. (2002) J Biol Chem. 277(3): 2112-2117. 4. Kokhan V.S., Afanasyeva M.A., Van'kin G. (2012) Behav. Brain. Res. 231(1): 226-230. 5. Spillantini M.G., et al. (1997) Nature. 388(6645): 839-840. 6. Mezey E., et al. (1998) Nat Med. 4(7): 755-757. 7. Polymeropoulos, M. H. (1998). Science. 276(5321), 2045–2047 8. Conway, K.E., et al. (1998). Nat Med. 4(11):1318-20 |
Product Images
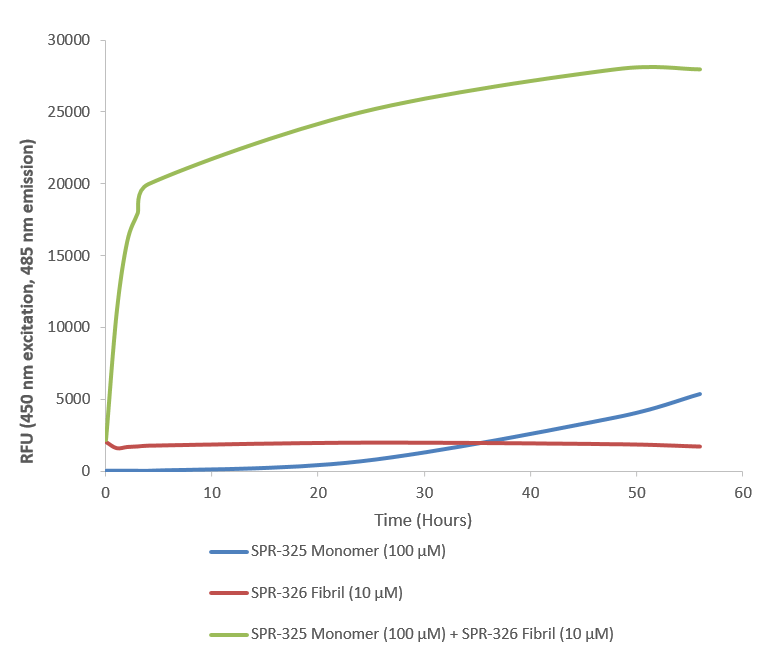
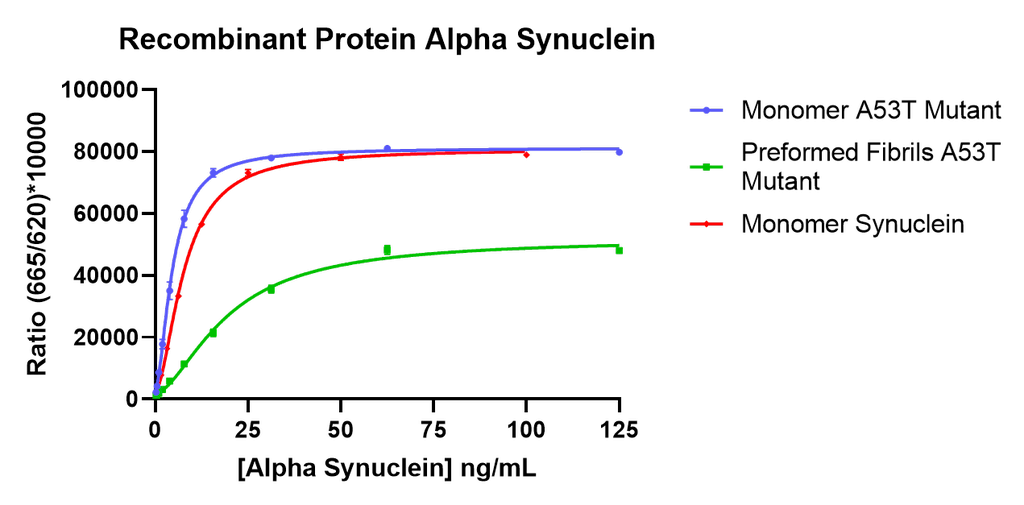
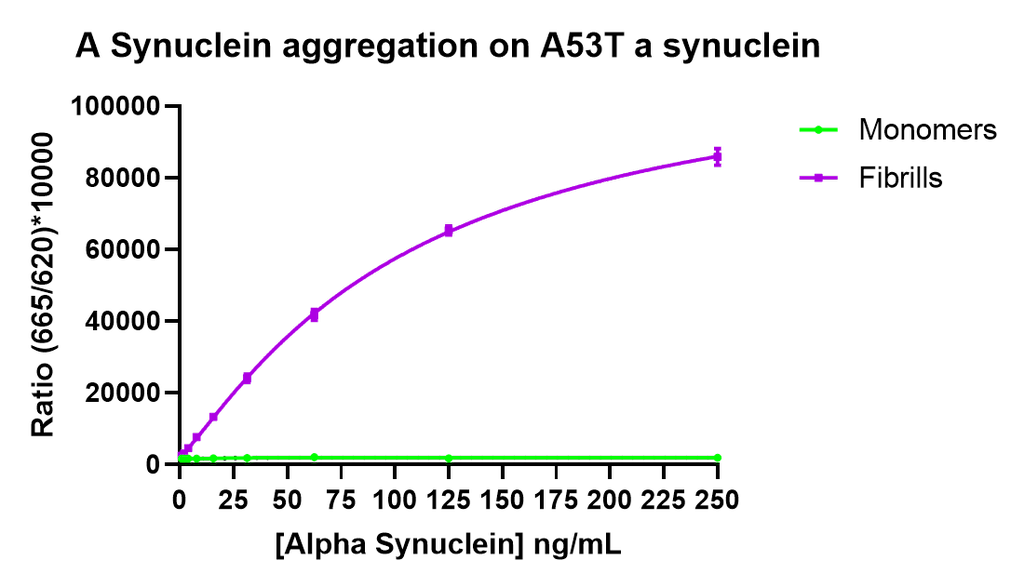
Thioflavin T is a fluorescent dye that binds to beta sheet-rich structures such as those in alpha synuclein fibrils. Upon binding, the emission spectrum of the dye experiences a red-shift and increased fluorescence intensity. Thioflavin T emission curves show a limited increase in fluorescence (correlated to alpha synuclein aggregation) over time in A53T alpha synuclein monomers (SPR-325). A much greater increase in fluorescence is seen when 100 µM monomer (SPR-325) is combined with 10 µM of fibrils (SPR-326) as the fibrils seed the formation of new fibrils from the pool of active monomers. Thioflavin T ex = 450 nm, em = 485 nm. Note: We use molecular weight of 14.46 kDa for both alpha synuclein monomer and fibril in calculations. We load 100µL/well for Thioflavin T assay so 100 µM is 144.6µg/well and 10 µM is 14.46 µg/well.






















StressMarq Biosciences :
Based on validation through cited publications.