Properties
| Storage Buffer | 20mM Tris/HCl, pH 7.5, 0.45M NaCl, 10% glycerol, 5mM bMe |
| Storage Temperature | -20ºC |
| Shipping Temperature | Blue Ice or 4ºC |
| Purification | Multi-Step Purified |
| Cite This Product | Bacteria Recombinant HSP65 Protein (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-116) |
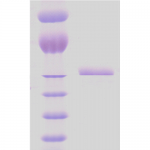
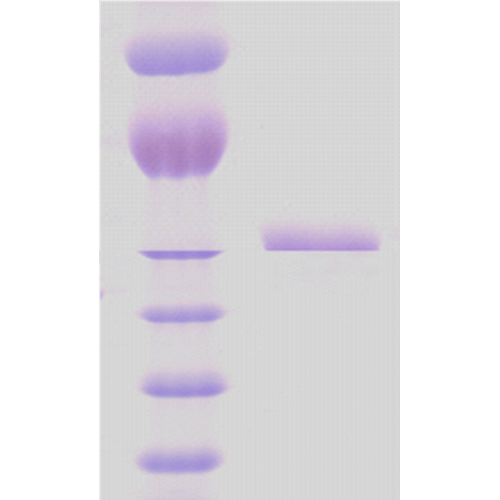
| Certificate of Analysis | This product has been certified >90% pure using SDS-PAGE analysis. |
Biological Description
| Alternative Names | 60kDa chaperonin 2, Antigen A, Cell wall A, groEL, GroEL2, GroL2, M. Tuberculosis cell wall A, M. Tuberculosis HSP65, Cpm60 2 |
| Research Areas | Cancer, Heat Shock |
| Cellular Localization | Cytoplasm |
| Accession Number | M15467 |
| Swiss Prot | P0A521 |
| Scientific Background |
HSP65, a member of the HSP60 family, is a mitochondrial chaperonin originally identified in Mycobacterium bovis BCG. It shares high sequence homology with HSP60 proteins across species and plays a central role in protein folding, particularly in the mitochondrial matrix. Like other chaperonins, HSP65 assists in the ATP-dependent folding and assembly of polypeptides, ensuring proper protein conformation and cellular function. While HSP65 is most commonly studied in the context of infectious disease and immunology—particularly as an immunodominant antigen in tuberculosis and leprosy—its structural and functional similarity to mammalian HSP60 has drawn attention in neurodegenerative disease research. Mitochondrial dysfunction and impaired proteostasis are key features of neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and multiple sclerosis. Given its chaperone activity, HSP65 serves as a model for understanding mitochondrial stress responses and protein misfolding in neurons. Moreover, HSP65 has been implicated in autoimmune responses, including experimental models of neuroinflammation. Its ability to trigger immune activation and molecular mimicry may contribute to the development of neuroimmune disorders, offering insights into the intersection of infection, immunity, and neurodegeneration. As a result, HSP65 is increasingly recognized not only as a microbial antigen but also as a valuable tool for studying mitochondrial resilience, neuroinflammation, and protein misfolding in the context of neurodegenerative disease. |
| References |
1. Koll H., et al. (1992) Cell. 68: 1163-1175. 2. Thole J.E.R., et al. (1985) Infect. Immuno. 50: 800-806. 3. Thole J.E.R., et al., (1987) Infect. Immuno. 55: 1466-1475. 4. Shinnick T.M. Sweetser D., Thole J., van Embden J. and Young R.A. (1987) Infect. Immuno. 55: 1932-1935. 5. Van Eden W., et al. (1988) Nature 331: 171-178. 6. Cobelens P.M., et al. (2002) Rheumatology 41: 775-779. |





















StressMarq Biosciences :
Based on validation through cited publications.