Properties
| Storage Buffer | PB pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Tau-441 (2N4R) Wild-Type Oligomers (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-497) |
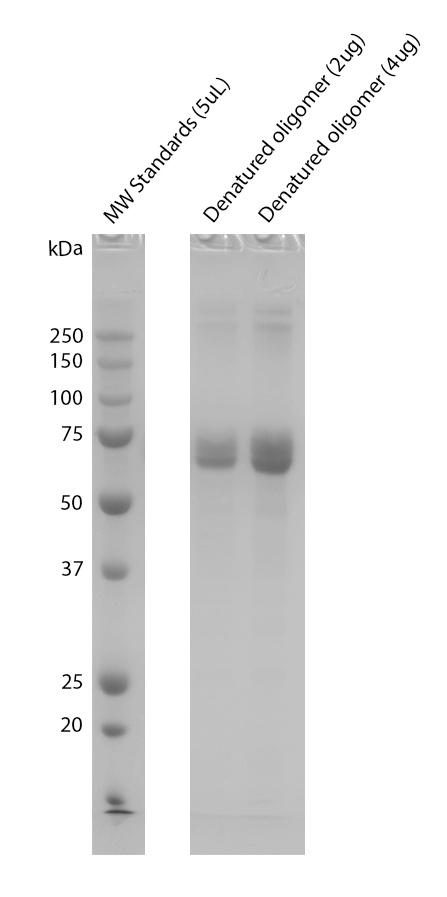
| Certificate of Analysis | Certified >95% pure using SDS-PAGE and A260/A280 analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | Monomer source is catalog# SPR-496. For corresponding PFFs, see catalog# SPR-498. |
Biological Description
| Alternative Names | MAPT, intracellular neurofibrillary tangles, NFTs, paired helical filaments, PHFs, 2N4R |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Tangles & Tau |
| Swiss Prot | P10636-8 |
| Scientific Background | Tau (tubulin-associated unit) is normally located in the axons of neurons where it stabilizes microtubules. Tauopathies such as Alzheimer’s Disease (AD) are characterized by neurofibrillary tangles containing hyper-phosphorylated tau fibrils (1) with consensus that tau oligomers are the most toxic species initiating neurodegeneration (2). Hyper-phosphorylated tau can be generated via expression in the Sf9/Baculovirus system, with up to 20 sites confirmed by mass spectrometry and western blots with phospho-specific antibodies (3). Our Sf9/Baculovirus-expressed Tau 2N4R oligomers are formed without the use of heparin or another anionic scaffold. |
| References |
1.,Iqbal K., Liu F., and Gong C.X. 2016. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. DOI: 10.1038/nrneurol.2015.225 2.,Puangmalai, N., Bhatt, N., Montalbano, M. et al. 2020. Internalization mechanisms of brain-derived tau oligomers from patients with Alzheimer’s disease, progressive supranuclear palsy and dementia with Lewy bodies. Cell Death Dis. DOI: 10.1038/s41419-020-2503-3 3.,Tepper et al. 2014. Oligomer Formation of Tau Protein Hyperphophorylated in Cells. The Journal of Biological Chemistry, DOI: 10.1074/jbc.M114.611368 |
Product Images
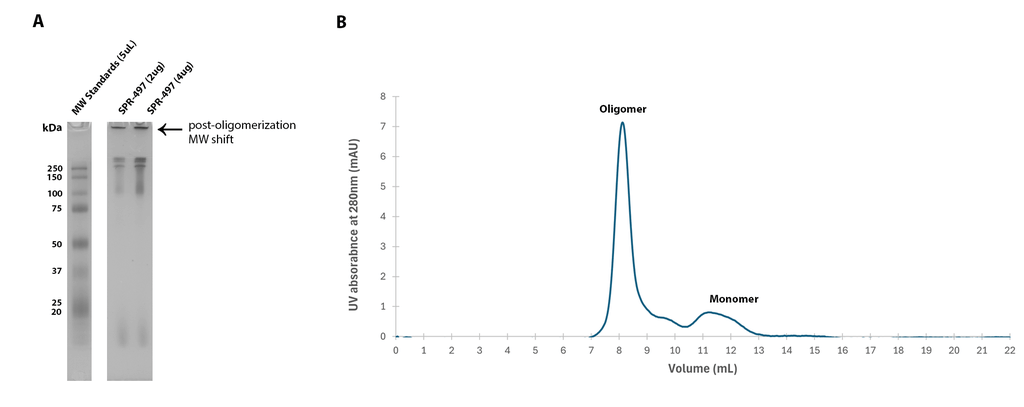
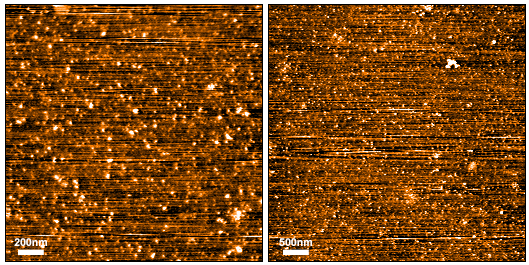
Post-oligomerization molecular weight (MW) shift of SPR-497 oligomers observed on a (A) 12% Tris-Glycine native-PAGE and by (B) size-exclusion chromatography of SPR-497. By peak area, at least 80% of SPR-497 is oligomeric. SEC was performed on Superdex 200 10/300 GL Increase column in phosphate buffer pH 7.4. Note: Monomeric Tau 2N4R is an intrinsically disordered 45 kDa protein. Due to its extended conformation in solution, migration of free monomeric Tau 2N4R is similar to that of a globular 220 kDa protein on Native PAGE and SEC.
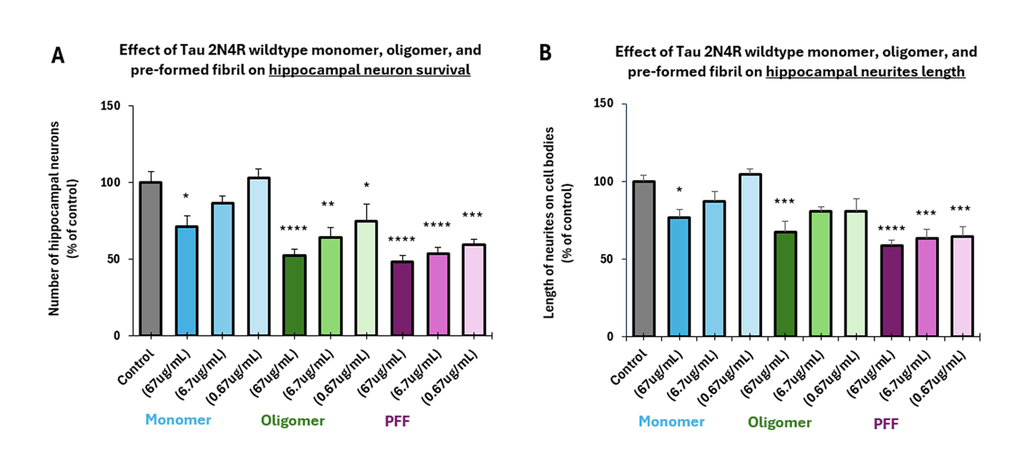
Tau 2N4R oligomers (catalog # SPR-497) and fibrils (catalog # SPR-498) show a dose-dependent toxicity to primary rat hippocampal neurons. Survival of rat primary hippocampal neurons 14 days after treatment with different concentrations of (A) monomers, (B) oligomers or (C) fibrils quantified by MAP2 positive neurons and expressed as a percentage of control. Fibrils were initially sonicated in a Bioruptor. Test conditions were run in the same plate as untreated control, which consisted of buffer without Tau protein. Data expressed as mean +/- s.e.m. (n=6). A global analysis of the data was performed using a one-way ANOVA followed by Dunnett’s test; * p < 0.05, ** p < 0.01, *** p < 0.0002, **** p < 0.0001; stats vs control.
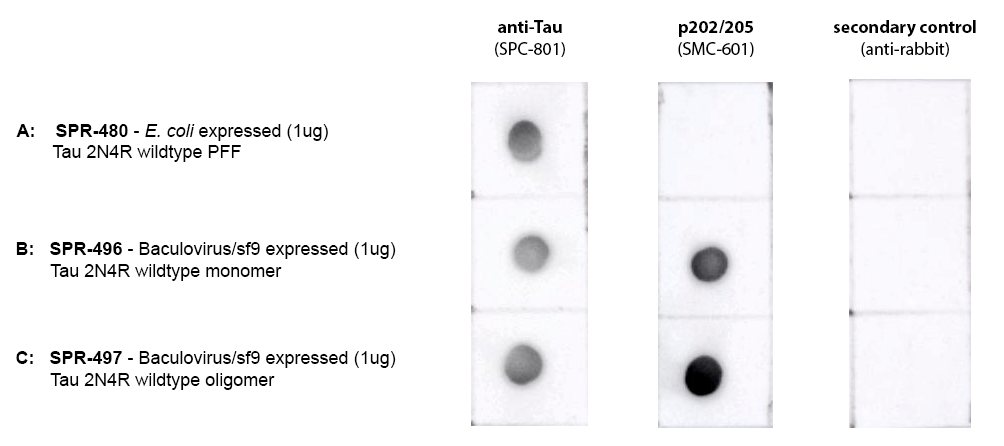
Dot Blot of purified hTau 2N4R constructs (SPR-480, SPR-496, SPR-497) using Stressmarq’s SPC-801 (anti-Tau) and SMC-601 (anti-p202/205) comparing phosphorylation in E.coli-expressed and baculovirus/sf9-expressed material. Protein was blotted on nitrocellulose, incubated with 1:1000 primary antibodies and/or 1:4000 secondary antibodies. Secondary control is goat-anti rabbit:HRP. Exposed 1 second.





















Reviews
There are no reviews yet.