| Product Name | Naloxone HCl |
| Description |
Opioid Receptor Antagonist |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 357-08-4 |
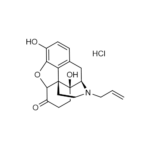
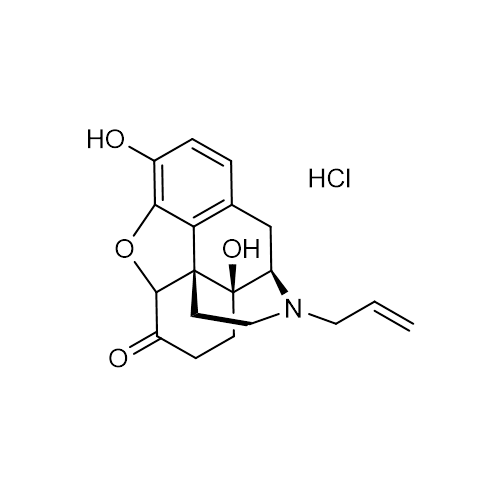
| Molecular Formula | C19H21NO4 • HCl |
| Molecular Weight | 363.8 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Antagonist |
| Solubility | May be dissolved in DMSO (35 mg/ml); water (30 mg/ml) |
| Source | Synthetic |
| Appearance | White crystalline powder |
| SMILES | C=CCN1CCC23C4C(=O)CCC2(C1CC5=C3C(=C(C=C5)O)O4)O.Cl |
| InChI | InChI=1S/C19H21NO4.ClH/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11;/h2-4,14,17,21,23H,1,5-10H2;1H/t14-,17+,18+,19-;/m1./s1 |
| InChIKey | RGPDIGOSVORSAK-STHHAXOLSA-N |
| Safety Phrases |
Classification: Warning. Hazard Statements: H302 Precautionary Statements: P264 - P270 - P301 + P312 - P501 |
| Cite This Product | Naloxone HCl (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-620) |
Biological Description
| Alternative Names | 5α)-4,5-Epoxy-3,14-dihydro-17-(2-propenyl)morphinan-6-one, hydrochloride |
| PubChem ID | 5464092 |
| Scientific Background | Naloxone HCl is a pan-opioid receptor antagonist widely used in clinical settings for opioid overdose reversal. Beyond its emergency applications, Naloxone is being explored in neuroscience for its ability to modulate opioid receptor-mediated signaling. It has been studied in models of pruritus, wound healing, and neuroimmune interactions, offering insights into the role of opioid receptors in neuroinflammation and neurodegenerative disease mechanisms. |
| References |
1. Le Bourdonnec B.,et al. (2008)Bioorg. Med. Chem. Lett. 18:2006. 2. EW., Boyer (2012) New Engl. J. Med. 367:146. 3. WangY., et al. (2017) Transl. Res. 185:13. 4. Wright FL., and RJ Rodgers (2013) Psychopharmacology (Berl.) 226:415. |

Reviews
There are no reviews yet.