| Product Name | C16 |
| Description |
PKR kinase inhibitor |
| Purity | >98% (HPLC) |
| CAS No. | 608512-97-6 |
| Molecular Formula | C13H8N4OS |
| Molecular Weight | 268.3 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in DMSO 12 mg/ml |
| Source | Synthetic |
| Appearance | Solid |
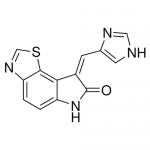
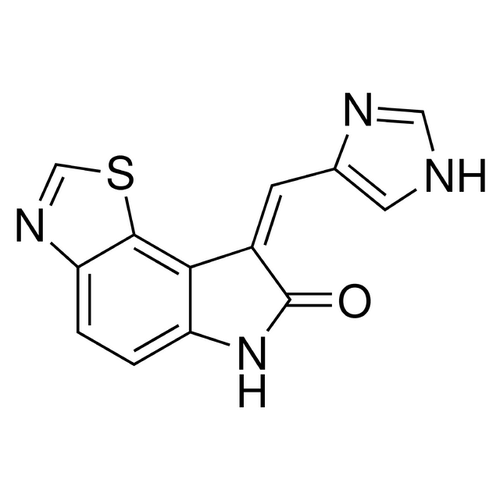
| SMILES | C1=CC2=C(C3=C1NC(=O)/C3=CC4=CNC=N4)SC=N2 |
| InChI | InChI=1S/C13H8N4OS/c18-13-8(3-7-4-14-5-15-7)11-9(17-13)1-2-10-12(11)19-6-16-10/h1-6H,(H,14,15)(H,17,18)/b8-3+ |
| InChIKey | VFBGXTUGODTSPK-FPYGCLRLSA-N |
| Safety Phrases |
Classification: Chronic aquatic toxicity (Category 4), H413 Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. Hazard statements: H413 May cause long lasting harmful effects to aquatic life. Precautionary statements: P273 Avoid release to the environment. P501 Dispose of contents/ container to an approved waste disposal plant. |
| Cite This Product | C16 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-498) |
Biological Description
| Alternative Names | Imidazolo-oxindole PKR inhibitor C16, 6,8-Dihydro-8-(1H-imidazol-5-ylmethylene)-7H-pyrrolo[2,3-g]benzothiazol-7-one |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, Cell Signaling, Tyrosine Kinase Inhibitors |
| PubChem ID | 67016828 |
| Scientific Background | C16 is a derivative of imidazolo-oxindole and functions as a selective inhibitor of RNA-dependent protein kinase (PKR), a key regulator of cellular stress responses. By targeting the ATP-binding site of PKR, C16 effectively blocks the PKR/eIF2α signaling pathway, which is implicated in apoptosis and neuroinflammation. In neurodegenerative disease research, C16 has gained attention for its potential to mitigate neuronal cell death and reduce inflammatory cascades associated with conditions such as Alzheimer’s and Parkinson’s disease. Its ability to modulate stress-induced translational control makes it a valuable tool for studying neuroprotective mechanisms and therapeutic interventions in central nervous system disorders. |
| References |
1. Couturier J., et al. (2010) J. Biol. Chem. 285(2): 1272–1282. 2. Jammi N.V., Whitby L.R., & Beal P.A. (2003) Biochem. Biophys.Res. Comm. 308(1):50–57. |

Reviews
There are no reviews yet.