| Product Name | Dimethyloxaloylglycine (DMOG) |
| Description |
Prolyl-4-hydroxylase inhibitor |
| Purity | >99% |
| CAS No. | 89464-63-1 |
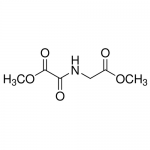
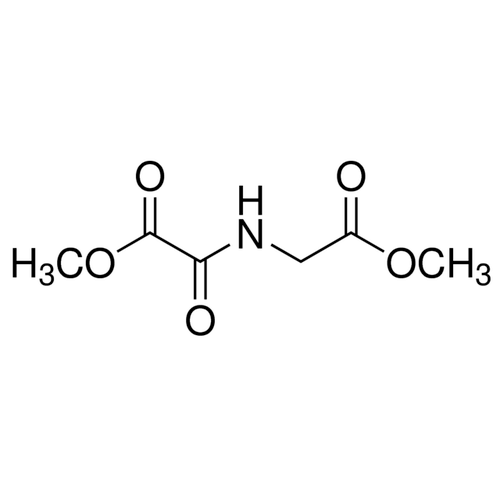
| Molecular Formula | C6H9NO5 |
| Molecular Weight | 175.1 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in DMSO (>25 mg/ml), 100% ethanol (>25 mg/ml) or dimethyl formamide, or PBS pH7.2 (10 mg/ml) |
| Source | Synthetic |
| Appearance | Off-white solid |
| SMILES | C(C(=O)OC)NC(C(=O)OC)=O |
| InChI | InChI=1S/C6H9NO5/c1-11-4(8)3-7-5(9)6(10)12-2/h3H2,1-2H3,(H,7,9) |
| InChIKey | BNJOZDZCRHCODO-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution. Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25 - Avoid contact with skin and eyes Hazard Statements: H302 - Harmful if swallowed |
| Cite This Product | Dimethyloxaloylglycine (DMOG) (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-382) |
Biological Description
| Alternative Names | DMOG, N-(Methoxyoxoacetyl)-glycine methyl ester |
| Research Areas | Cell Signaling |
| PubChem ID | 560326 |
| Scientific Background | DMOG is a cell-permeable inhibitor of prolyl-4-hydroxylase and factor inhibiting HIF (FIH), leading to stabilization and activation of hypoxia-inducible factor (HIF). In neuroscience, DMOG is used to model hypoxic conditions and to study HIF-mediated neuroprotection, angiogenesis, and metabolic adaptation. HIF activation has been linked to enhanced neuronal survival, reduced oxidative stress, and improved vascularization—mechanisms relevant to stroke, traumatic brain injury, and neurodegenerative diseases. DMOG’s ability to upregulate HIF signaling in vivo and attenuate ischemic injury makes it a powerful compound for investigating therapeutic strategies targeting brain hypoxia and mitochondrial dysfunction. Its pro-angiogenic properties also support research into neurovascular remodeling and blood-brain barrier integrity. |
| References |
1. Grozinger C.M., et al. (2001) J. Biol. Chem. 276(42): 38837-43. 2. Mai A., et al. (2005) J. Med. Chem. 48(24): 7789-95. 3. Kahyo T., et al. (2008) J. Pharmacol. Sci. 108(3): 364-71. 4. Jung-Hynes B., et al. (2009) J. Biol. Chem. 284(6): 3823-32. |

Reviews
There are no reviews yet.