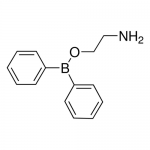
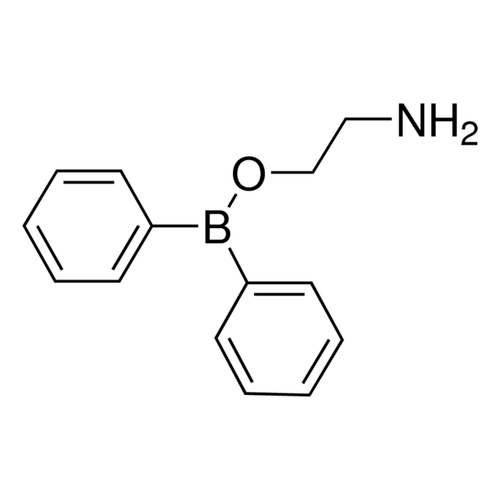
| Product Name | 2-APB |
| Description |
TRP blocker |
| Purity | >98% |
| CAS No. | 524-95-8 |
| Molecular Formula | C14H16BNO |
| Molecular Weight | 225.1 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol |
| Source | Synthetic |
| Appearance | White solid |
| SMILES | B(C1=CC=CC=C1)(C2=CC=CC=C2)OCCN |
| InChI | InChI=1S/C14H16BNO/c16-11-12-17-15(13-7-3-1-4-8-13)14-9-5-2-6-10-14/h1-10H,11-12,16H2 |
| InChIKey | BLZVCIGGICSWIG-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Hazard Phrases: H302-H315-H317-H318-H335 Precautionary Phrases: P261-P280-P305 + P351 + P338 |
| Cite This Product | 2-APB (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-316) |
Biological Description
| Alternative Names | 2-Aminoethoxydiphenylborane |
| Research Areas | Ion Channels, Neuroscience |
| PubChem ID | 1598 |
| Scientific Background | 2-APB is a versatile chemical tool that inhibits IP3 receptors and modulates TRP channel activity. In neuroscience, it is widely used to manipulate intracellular calcium release and study calcium-dependent signaling pathways. 2-APB also inhibits gap junctions composed of connexin26 and connexin32, making it relevant in research on neuronal communication and network synchronization. Its broad activity profile supports its use in studies of neurodevelopment, synaptic plasticity, and neurodegeneration. |
| References |
1. Diver J.M., Sage S.O., Rosado J.A. (2001) Cell Calcium. 30(5): 323-329 2. Xu S.Z., et al. (2005) Br J Pharamcol. 145(4): 320-328. 3. Tao L., and Harris A.L. (2007) Mol Pharmacol. 71(2): 570-579. |

StressMarq Biosciences :
Based on validation through cited publications.