| Product Name | Astemizole |
| Description |
Herg channel blocker |
| Purity | >98% |
| CAS No. | 68844-77-9 |
| Molecular Formula | C28H31FN4O |
| Molecular Weight | 458.57 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol |
| Source | Synthetic |
| Appearance | White solid |
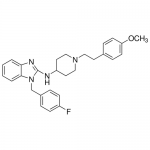
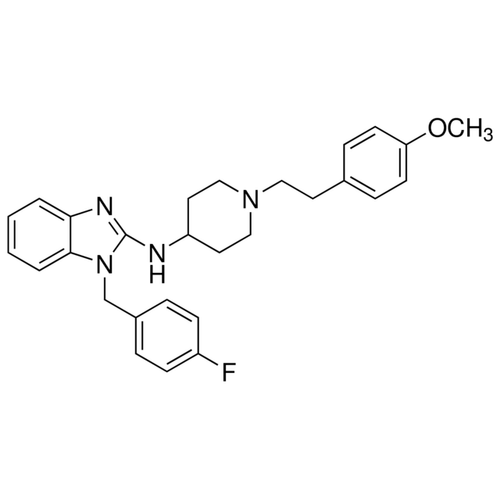
| SMILES | COC1=CC=C(C=C1)CCN2CCC(CC2)NC3=NC4=CC=CC=C4N3CC5=CC=C(C=C5)F |
| InChI | InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-2 |
| InChIKey | GXDALQBWZGODGZ-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Risk Phrases: R62 - Possible risk of impaired fertility Hazard Phrases: H315-H319-H335 Precautionary Phrases: P261-P305 + P351 + P338 |
| Cite This Product | Astemizole (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-302) |
Biological Description
| Alternative Names | 1-(4-Fluorobenzyl)-N-{1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl}-1H-benzimidazol-2-amine, Hismanal |
| Research Areas | Ion Channels, Neuroscience |
| PubChem ID | 2247 |
| Scientific Background | Astemizole is a second-generation antihistamine that binds to peripheral histamine H1 receptors in tissues such as the gastrointestinal tract, uterus, and bronchial muscle. Although it does not cross the blood-brain barrier, its peripheral selectivity makes it useful in studying histamine-related signaling and its indirect effects on the nervous system. It is metabolized by CYP3A4 and has been explored for its off-target effects in neuropharmacology. |
| References |
1. Laduron P.M., Janssen P.F., Commeren W. and Leysen J.E. (1982) Mol Pharmacol. 21(2): 294-300. 2. Maysumoto S., Yamazoe Y. (2001) Br. J Clin Pharamcol. 51(2): 133-142. |

Reviews
There are no reviews yet.