| Product Name | Baricitinib |
| Description |
Kinase inhibitor |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 1187594-09-7 |
| Molecular Formula | C16H17N7O2S |
| Molecular Weight | 371.4 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (30 mg/ml); or DMF (50 mg/ml) |
| Source | Synthetic |
| Appearance | White to off-white powder |
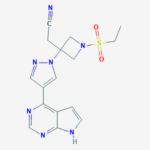
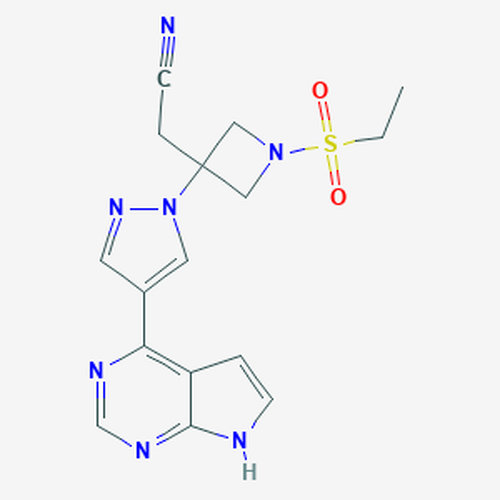
| SMILES | CCS(=O)(=O)N1CC(C1)(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3 |
| InChI | InChI=1S/C16H17N7O2S/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20) |
| InChIKey | XUZMWHLSFXCVMG-UHFFFAOYSA-N |
| Safety Phrases |
Hazard statements Harmful if swallowed. · Precautionary statements Wash thoroughly after handling. Do not eat, drink or smoke when using this product. |
| Cite This Product | Baricitinib (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-587) |
Biological Description
| Alternative Names | 1-(Ethylsulfonyl)-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]-3-azetidineacetonitrile, INCB028050, LY3009104 |
| Research Areas | Cell Signaling, Growth Factors, TNF |
| PubChem ID | 44205240 |
| Scientific Background |
Baricitinib is a selective Janus kinase (JAK1/JAK2) inhibitor that modulates immune signaling by suppressing cytokine release. While primarily used in autoimmune diseases and COVID-19-related cytokine storm management, Baricitinib is gaining interest in neuroinflammation research. Chronic activation of JAK-STAT signaling is implicated in neurodegenerative diseases such as multiple sclerosis and Alzheimer’s. By dampening pro-inflammatory cytokine cascades, Baricitinib may offer neuroprotective benefits and is being explored as a therapeutic candidate for CNS disorders involving immune dysregulation and glial activation. |
| References | 1."Summary of opinion for Olumiant" (PDF). European Medicines Agency (EMA). 15 December 2016. 2. Cantini F., et al. (2020) J. Infect. DOI: 10.1016/j.jinf.2020.04.017 |

Reviews
There are no reviews yet.