| Product Name | Iniparib |
| Description |
PARP1 inhibitor |
| Purity | >98% |
| CAS No. | 160003-66-7 |
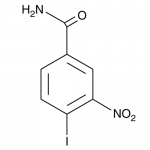
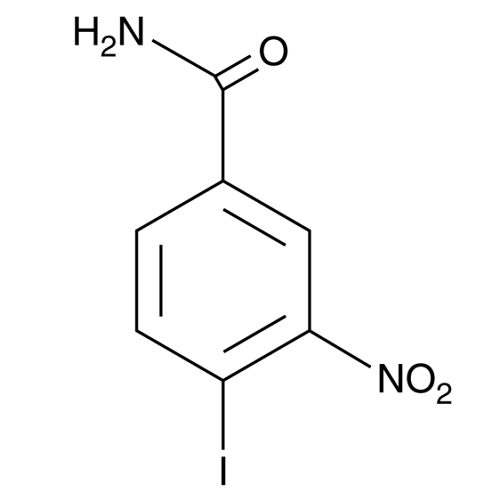
| Molecular Formula | C7H5IN2O3 |
| Molecular Weight | 292 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in DMSO (5 mg/ml) |
| Source | Synthetic |
| Appearance | White to beige powder |
| SMILES | C1=CC(=C(C=C1C(=O)N)[N+](=O)[O-])I |
| InChI | 1S/C7H5IN2O3/c8-5-2-1-4(7(9)11)3-6(5)10(12)13/h1-3H,(H2,9,11) |
| InChIKey | MDOJTZQKHMAPBK-UHFFFAOYSA-N |
| Safety Phrases |
Classification: D2B Toxic Material Causing Other Toxic Effects - Moderate eye irritant Hazard statement(s): H302 Harmful if swallowed. H319 Causes serious eye irritation. Precautionary statement(s): P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| Cite This Product | BSI 201 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-539) |
Biological Description
| Alternative Names | Iniparib, 4-Iodo-3-nitrobenzamide, BSI-201, 4-iodo-3-nitro-benzamide, Iniparib (BSI-201) |
| Research Areas | Cancer, Cancer Growth Inhibitors |
| PubChem ID | 9796068 |
| Scientific Background | BSI 201 is a small molecule inhibitor of poly(ADP-ribose) polymerase 1 (PARP1), an enzyme involved in DNA repair and cellular stress responses. While widely studied in oncology for its ability to induce synthetic lethality in tumor cells with DNA repair deficiencies, PARP1 inhibition is also relevant to neurodegenerative disease research. Excessive PARP1 activation has been linked to neuronal death in conditions such as stroke, Parkinson’s disease, and Alzheimer’s disease. By modulating DNA repair and inflammatory pathways, BSI 201 may help mitigate neuronal damage and promote neuroprotection. Its dual relevance to cancer and neuroscience underscores its value in translational research. |

Reviews
There are no reviews yet.