| Product Name | Camostat mesylate |
| Description |
Serine protease inhibitor |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 59721-29-8 |
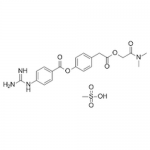
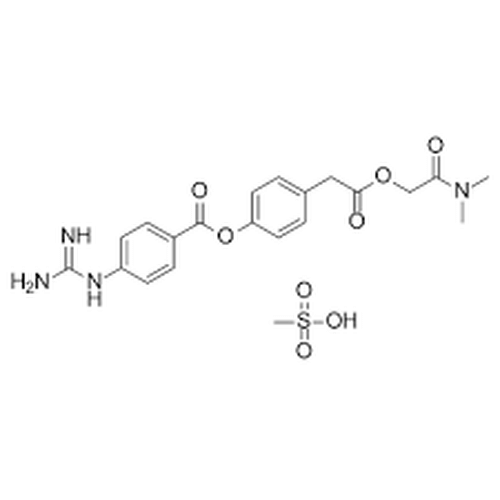
| Molecular Formula | C20H22N4O5·CH3SO3H |
| Molecular Weight | 494.5 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (50 mg/mL) or Water (50mg/mL) |
| Source | Synthetic |
| Appearance | Off-white powder |
| SMILES | O=C(C2=CC=C(NC(N)=N)C=C2)OC1=CC=C(CC(OCC(N(C)C)=O)=O)C=C1.CS(=O)(O)=O |
| InChI | InChI=1S/C20H22N4O5.CH4O3S/c1-24(2)17(25)12-28-18(26)11-13-3-9-16(10-4-13)29-19(27)14-5-7-15(8-6-14)23-20(21)22;1-5(2,3)4/h3-10H,11-12H2,1-2H3,(H4,21,22,23);1H3,(H,2,3,4) |
| InChIKey | FSEKIHNIDBATFG-UHFFFAOYSA-N |
| Safety Phrases | General Classification: Warning Hazard statements: H315-H319-H335-H400 Precautionary statements: P261-P273-P305 + P351 + P338 |
| Cite This Product | Camostat mesylate (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-585) |
Biological Description
| Alternative Names | 4-[[4-[(Aminoiminomethyl)amino]benzoyl]oxy]benzeneacetic acid 2-(dimethylamino)-2-oxoethyl ester methanesulfonate; FOY 305; FOY-S 980; Foipan mesylate |
| Research Areas | Cancer, Cell Signaling |
| PubChem ID | 5284360 |
| Scientific Background |
Camostat mesylate is an orally bioavailable serine protease inhibitor best known for its inhibition of TMPRSS2, a key enzyme facilitating viral entry in respiratory infections such as COVID-19. While its primary research focus lies in infectious disease and fibrosis, Camostat’s modulation of protease activity has implications for neuroinflammation and blood-brain barrier integrity. Emerging studies suggest potential roles in neurodegenerative disease models where protease dysregulation contributes to pathology. Its anti-fibrotic and anti-inflammatory properties also make it a candidate for repurposing in neurological contexts involving chronic inflammation or glial activation. |
| References |
1. Okuno M., et al. (2002). Front Biosci. 7 (4): d204-218. 2. Hsieh H. P., Hsu, J. T. (2007). Current Pharmaceutical Design. 13 (34): 3531–3542. 3. Kitamura K., Tomita K. (2012). Clinical and Experimental Nephrology. 16 (1): 44–48. 4. Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. (2012) J Virol. 86(12): 6537-6545. 5.. Hoffman M., et al. (2020) Cell: https://doi.org/10.1016/j.cell.2020.02.052 |

Reviews
There are no reviews yet.