| Product Name | Cytochalasin D |
| Description |
Actin polymerization inhibitor |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Solubility | Soluble to 10 mM in ethanol and to 50 mM in DMSO |
| Appearance | White Solid |
| Safety Phrases |
Classification: Toxic. May be harmful or fatal if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Risk Phrases: R62 - Possible risk of impaired fertility R68 - Possible risk of irreversible effects Hazard Phrases: H300-H361 Precautionary Phrases: P264-P281-P301 + P310 |
| Cite This Product | Cytochalasin D (StressMarq Biosciences, Canada, Cat # SIH-241) |
Biological Description
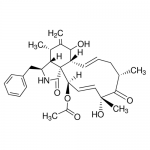
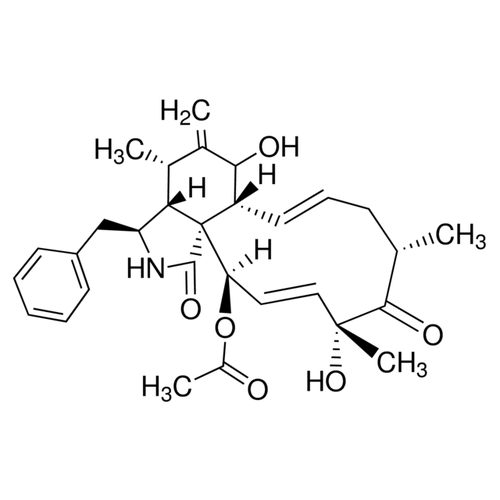
| Alternative Names | (3S,3aR,4S,6S,6aR,7E,10S,12S,13E,15R,15aR)-3-Benzyl-6-hydroxy-4,10,12-trimethyl-5-methylene-1-oxo-2,3,3a,4,5,6,6a,9,10,11,12,15-dodecahydro-1H-cycloundeca[d]isoindol-15-yl acetate, Zygosporin A |
| Scientific Background | Cytochalasin D binds to actin filaments and inhibits their polymerization and elongation, leading to changes in cellular morphology and apoptosis. In neuroscience, it is widely used to study cytoskeletal dynamics, synaptic plasticity, and neuronal migration. Its ability to disrupt actin networks makes it a powerful agent for probing the role of the cytoskeleton in neurodevelopment and neurodegeneration. |
| References |
1. Haidle A., and Myers A. (2004) Proc Natl Acad Sci. 101(33): 12048-12053. 2. Ornelles et al., (1986) Mol Cell Biol. 6(5): 1650-1662. |

StressMarq Biosciences :
Based on validation through cited publications.