| Product Name | CZC 54252 Hydrochloride |
| Description |
LRRK2 inhibitor |
| Purity | >98% |
| CAS No. | 1191911-27-9, 1330003-04-7 |
| Molecular Formula | C22H26Cl2N6O4S |
| Molecular Weight | 541.5 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 100 mM in DMSO |
| Source | Synthetic |
| Appearance | Yellow solid |
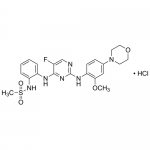
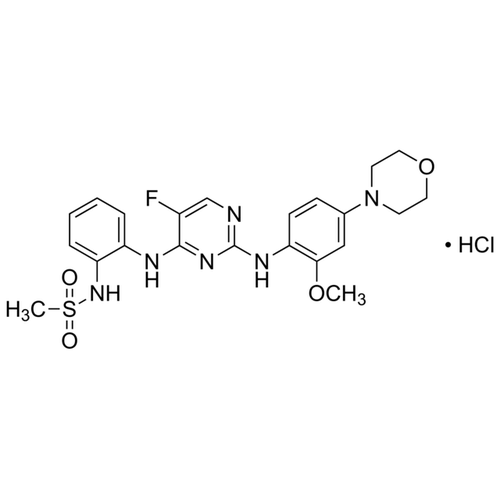
| SMILES | COC1=C(C=CC(=C1)N2CCOCC2)NC3=NC=C(C(=N3)NC4=CC=CC=C4NS(=O)(=O)C)Cl.Cl |
| InChI | InChI=1S/C22H25ClN6O4S.ClH/c1-32-20-13-15(29-9-11-33-12-10-29)7-8-19(20)26-22-24-14-16(23)21(27-22)25-17-5-3-4-6-18(17)28-34(2,30)31;/h3-8,13-14,28H,9-12H2,1-2H3,(H2,24,25,26,27);1H |
| InChIKey | KWCBHUPLQMUKAF-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Not a hazardous substance or mixture. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. |
| Cite This Product | CZC 54252 Hydrochloride (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-439) |
Biological Description
| Alternative Names | N-{2-[(5-Chloro-2-{[2-methoxy-4-(4-morpholinyl)phenyl]amino}-4-pyrimidinyl)amino]phenyl}methanesulfonamide hydrochloride |
| Research Areas | Cell Signaling, Neurodegeneration, Neuroscience, Multiple System Atrophy |
| Scientific Background | CZC 54252 Hydrochloride is a potent and selective inhibitor of leucine-rich repeat kinase 2 (LRRK2), a kinase genetically linked to familial and sporadic Parkinson’s disease. LRRK2 mutations are associated with altered kinase activity, leading to impaired autophagy, mitochondrial dysfunction, and neuroinflammation. CZC 54252 is widely used in Parkinson’s disease research to study the effects of LRRK2 inhibition on neuronal survival, protein clearance, and inflammatory responses. Its selectivity and potency make it a valuable tool for validating LRRK2 as a therapeutic target in neurodegeneration. |
| References | 1. Kramer T., Lo Monte F., Göring S., Okala Amombo G.M., & Schmidt B. (2012) ACS Chem. Neuro. 3(3): 151–160. |

Reviews
There are no reviews yet.