| Product Name | Dofetilide |
| Description |
Herg channel blocker |
| Purity | >98% |
| CAS No. | 115256-11-6 |
| Molecular Formula | C19H27N3O5S2 |
| Molecular Weight | 441.56 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 100 mM in DMSO |
| Source | Synthetic |
| Appearance | White Solid |
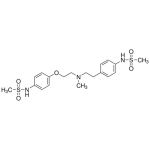
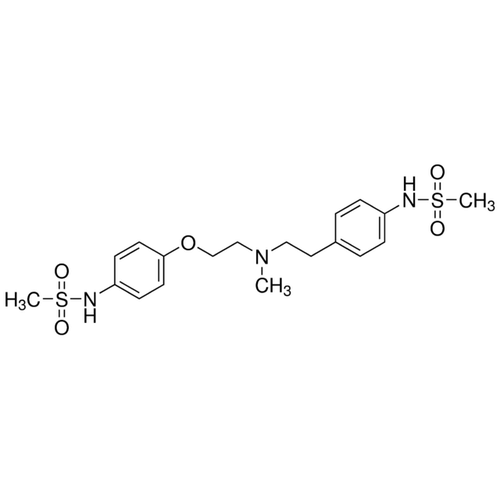
| SMILES | CN(CCC1=CC=C(C=C1)NS(=O)(=O)C)CCOC2=CC=C(C=C2)NS(=O)(=O)C |
| InChI | InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H |
| InChIKey | IXTMWRCNAAVVAI-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Toxic. May be harmful or fatal if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S53 - Avoid exposure - obtain special instruction before use S57 - Use appropriate containment to avoid environmental contamination Risk Phrases: R22 - Harmful if swallowed R48 - Danger of serious damage to health by prolonged exposure R51/53 - Toxic to aquatic organisms, may cause longterm adverse effects in the aquatic environment R61 - May cause harm to the unborn child R62 - Possible risk of impaired fertility Hazard Phrases: H302-H319-H360 Precautionary Phrases: P201-P305 + P351 + P338-P308 + P313 |
| Cite This Product | Dofetilide (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-311) |
Biological Description
| Alternative Names | N-(4-{2-[Methyl(2-{4-[(methylsulfonyl)amino]phenoxy}ethyl)amino]ethyl}phenyl)methanesulfonamide, Tikosyn, UK 68798 |
| Research Areas | Ion Channels, Neuroscience |
| PubChem ID | 71329 |
| Scientific Background | Dofetilide is a class III antiarrhythmic agent that selectively blocks the rapid component of the delayed rectifier potassium current. While primarily used in cardiology, its effects on potassium channels have relevance in neuroscience, particularly in studies of neuronal excitability and ion channel pharmacology. Understanding how dofetilide modulates potassium currents can inform research on seizure activity, neuroprotection, and the electrophysiological properties of neurons. |
| References |
1. Lentz T.L., Hilleman D.E. (2000) Pharmacotherapy. 20(7): 776-786. 2. Roukoz H., Saliba W. (2007) Expert Rev Cardiovasc Ther. 5(1): 9-19. |

Reviews
There are no reviews yet.