| Product Name | Epoxomicin |
| Description |
Proteasome inhibitor |
| Purity | >95% |
| CAS No. | 134381-21-8 |
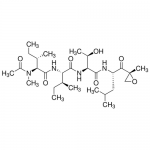
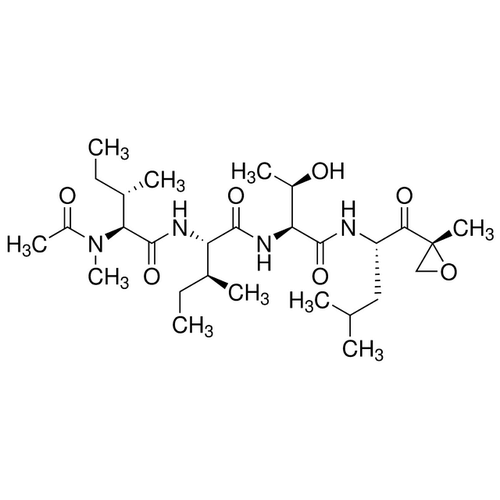
| Molecular Formula | C28H50N4O7 |
| Molecular Weight | 554.7 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in DMSO (15 mg/ml) or dichloromethane:methanol (9:1); insoluble in water. |
| Source | Synthetic |
| Appearance | White solid |
| SMILES | [C@@H](CC)(C)[C@@H](C(N[C@H](C(N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(=O)NCC(=O)[C@]1(C)OC1)CC(C)C)=O)[C@H](CC)C)=O)N(C)C |
| InChI | InChI=1S/C28H50N4O7/c1-11-16(5)21(30-27(38)23(17(6)12-2)32(10)19(8)34)25(36)31-22(18(7)33)26(37)29-20(13-15(3)4)24(35)28(9)14-39-28/h15-18,20-23,33H,11-14H2,1-10H3,(H,29,37)(H,30,38)(H,31,36)/t16-,17 |
| InChIKey | DOGIDQKFVLKMLQ-JTHVHQAWSA-N |
| Safety Phrases |
Classification: Caution. Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25 - Avoid contact with skin and eyes |
| Cite This Product | Epoxomicin (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-388) |
Biological Description
| Alternative Names | N-Acetyl-N-methyl-L-isoleucyl-L-isoleucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-L-threoninamide |
| Research Areas | Cell Signaling |
| PubChem ID | 11226684 |
| Scientific Background | Epoxomicin is a potent, cell-permeable, and selective inhibitor of the proteasome, originally isolated from an Actinomycetes strain for its antitumor properties. In neuroscience, Epoxomicin is widely used to study the ubiquitin-proteasome system (UPS), a critical pathway for protein degradation and cellular homeostasis in neurons. Dysfunction of the UPS is a hallmark of several neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and Huntington’s disease, where misfolded or aggregated proteins accumulate. Epoxomicin inhibits the chymotrypsin-like, trypsin-like, and PGPH activities of the proteasome, making it a valuable tool for dissecting proteostasis mechanisms. It also indirectly activates autophagy, a compensatory degradation pathway, by blocking UPS function. Additionally, Epoxomicin suppresses NF-κB activation and inflammation, further supporting its use in neuroinflammatory models. Its dual role in modulating protein clearance and inflammatory signaling makes it highly relevant in neurodegenerative disease research. |
| References |
1. F. Yang, et al. (2009) Neurosci. Lett. 454: 203. 2. K. Ohkawa, et al. (2004) Int. J. Oncol. 24: 425. 3. K. Schwarz, et al. (2000) J. Immunol. 164: 6147. 4. L. Meng, et al. (1999) PNAS. 96: 10403. 5. K.B. Kim, et al. (1999) Bioorg. Med. Chem. Lett. 9: 3335. 6. N. Sin, et al. (1999) Bioorg. Med. Chem. Lett. 9: 2283. 7. M. Hanada, et al. (1992) J. Antibiot. (Tokyo) 45: 1746. |

Reviews
There are no reviews yet.