| Product Name | Fasudil |
| Description |
ROCK inhibitor |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 103745-39-7 |
| Molecular Formula | C14H17N3O2S•HCl |
| Molecular Weight | 327.83 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (75 mg/ml) or water (100 mg/ml) |
| Source | Synthetic |
| Appearance | Powder |
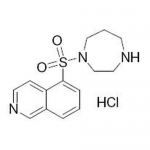
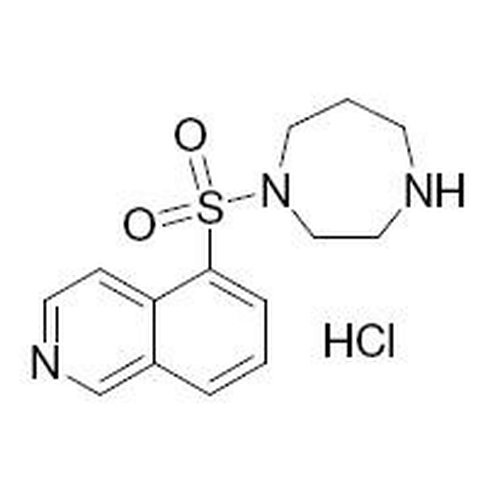
| SMILES | C1CNCCN(C1)S(=O)(=O)C2=CC=CC3=C2C=CN=C3 |
| InChI | InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 |
| InChIKey | NGOGFTYYXHNFQH-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | Fasudil (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-371) |
Biological Description
| Alternative Names | 1-(5-Isoquinolinylsulfonyl) homopiperazine hydrochloride; 5-(1,4-Diazepan-1-ylsulfonyl)isoquinoline |
| Research Areas | Cell Signaling, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein |
| PubChem ID | 3547 |
| Scientific Background | Fasudil is a selective Rho-associated protein kinase (ROCK2) inhibitor with additional activity on PRK2 and MSK1, and is gaining traction in neuroscience for its neuroprotective and vasodilatory effects. By modulating cytoskeletal dynamics and reducing neuroinflammation, Fasudil has demonstrated efficacy in models of stroke, spinal cord injury, and Alzheimer’s disease. Its ability to inhibit multiple kinases involved in neuronal signaling and vascular tone positions it as a promising candidate for treating neurovascular and neurodegenerative disorders. |
| References |
1. Shirotani M., et al. (1991) J. Pharmacol. Exp. Ther. 259(2): 738-744. 2. Arai M., Nozawa R., et al. (1993) Biochem. Pharmacol. 46(8): 1487-1490. 3. Satoh S., et al. (1996) Br. J. Pharmacol. 118(7): 1592-1606. 4. Sward K., et al. (2000) J. Physiol. 522: 33-49. |

Reviews
There are no reviews yet.