| Product Name | IOX1 |
| Description |
JMJD inhibitor |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 5852-78-8 |
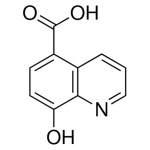
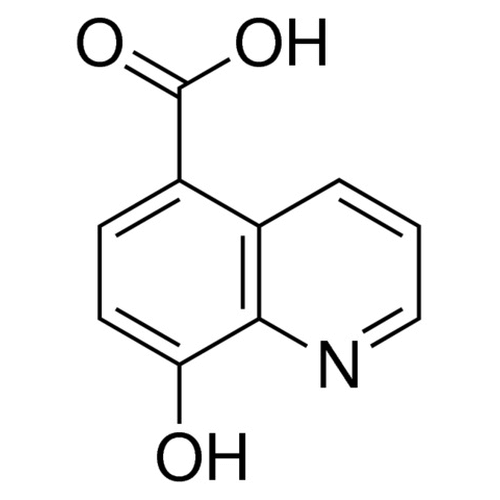
| Molecular Formula | C10H7NO3 |
| Molecular Weight | 189.2 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (20 mg/ml) |
| Source | Synthetic |
| Appearance | Yellow powder |
| SMILES | C1=CC2=C(C=CC(=C2N=C1)O)C(=O)O |
| InChI | InChI=1S/C10H7NO3/c12-8-4-3-7(10(13)14)6-2-1-5-11-9(6)8/h1-5,12H,(H,13,14) |
| InChIKey | JGRPKOGHYBAVMW-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | IOX1 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-377) |
Biological Description
| Alternative Names | 8-Hydroxy-5-quinolinecarboxylic acid; (5-Carboxy-8HQ) |
| Research Areas | Cell Signaling |
| PubChem ID | 459617 |
| Scientific Background | IOX1 is a broad-spectrum inhibitor of 2-oxoglutarate-dependent dioxygenases, including histone demethylases. In neuroscience, IOX1 is used to investigate epigenetic regulation of gene expression in neuronal cells. Epigenetic modifications play a crucial role in neurodevelopment, synaptic plasticity, and the pathogenesis of neurodegenerative diseases. By inhibiting histone demethylation, IOX1 alters chromatin structure and transcriptional activity, providing insights into the molecular mechanisms underlying memory formation, neuroinflammation, and neuronal survival. Its utility in epigenetic studies makes it a valuable tool for exploring therapeutic strategies targeting chromatin remodeling in the brain. |
| References | 1. King O.N., et al. (2010) PLoS One. 5: e15535. |

Reviews
There are no reviews yet.