| Product Name | Lestaurtinib |
| Description |
FLT3, TrkA kinase inhibitor |
| Purity | >98% (HPLC) |
| CAS No. | 111358-88-4 |
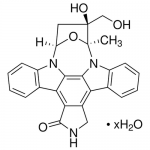
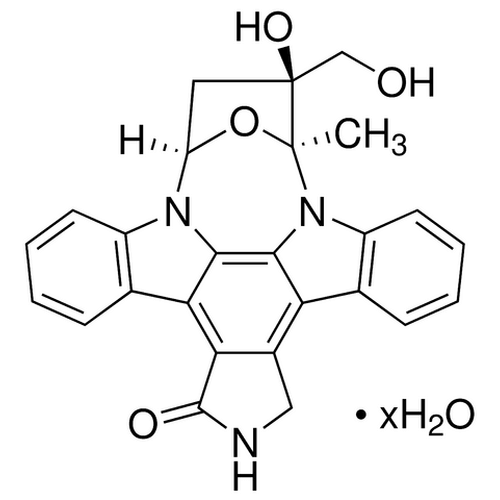
| Molecular Formula | C26H21N3O4 |
| Molecular Weight | 439.5 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol |
| Source | Synthetic |
| Appearance | White powder |
| SMILES | [C@@]67([N]2C1=C5C(=C4C(=C1C3=C2C=CC=C3)CNC4=O)C8=C([N]5[C@H](O6)C[C@]7(O)CO)C=CC=C8)C |
| InChI | InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 |
| InChIKey | UIARLYUEJFELEN-LROUJFHJSA-N |
| Safety Phrases |
Classification: Not a hazardous substance or mixture. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. |
| Cite This Product | Lestaurtinib (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-460) |
Biological Description
| Alternative Names | KT5555, CEP-701, (9S,10S,12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-10-(hydroxymethyl)-9-methyl-9,12-epoxy-1H-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazocin-1-one |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, Cell Signaling, Tyrosine Kinase Inhibitors |
| PubChem ID | 126565 |
| Scientific Background | Lestaurtinib is a potent inhibitor of JAK2, FLT3, and TrkA receptor tyrosine kinases. In neuroscience, it is used to study neurotrophic signaling and inflammatory pathways. By inhibiting TrkA, Lestaurtinib helps elucidate the role of nerve growth factor (NGF) signaling in neuronal survival and differentiation. Its inhibition of JAK2 also makes it relevant in models of neuroinflammation and cytokine signaling. Lestaurtinib is a valuable compound for investigating kinase-driven mechanisms in neurodegenerative diseases. |
| References |
1. Weisel K.C., et al. (2007) Ann. NY Acad. Sci. 1106: 190–196. 2. Hexner E.O., et al. (2008) Blood. 111(12): 5663–5671. |

Reviews
There are no reviews yet.