| Product Name | Myriocin (ISP-1) |
| Description |
Palmitoyltransferase inhibitor |
| Purity | >98% |
| CAS No. | 35891-70-4 |
| Molecular Formula | C21H39NO6 |
| Molecular Weight | 401.54 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in 2 mg/ml of methanol, DMSO (25 mg/ml; heat briefly in boiling water bath) or in dilute base (5 mg/ml; 50 mM NaOH) |
| Source | Synthetic |
| Appearance | Off-White Solid |
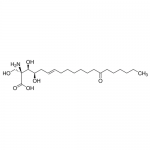
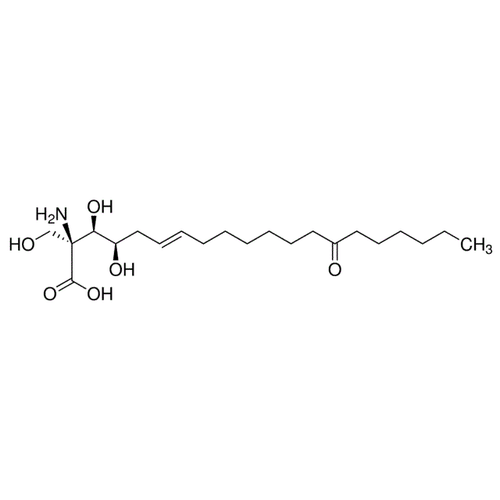
| SMILES | CCCCCCC(=O)CCCCCC/C=C/C[C@H]([C@@H]([C@@](CO)(C(=O)O)N)O)O |
| InChI | InChI=1S/C21H39NO6/c1-2-3-4-10-13-17(24)14-11-8-6-5-7-9-12-15-18(25)19(26)21(22,16-23)20(27)28/h9,12,18-19,23,25-26H,2-8,10-11,13-16,22H2,1H3,(H,27,28)/b12-9+/t18-,19+,21+/m1/s1 |
| InChIKey | ZZIKIHCNFWXKDY-GNTQXERDSA-N |
| Safety Phrases |
Classification: Harmful. May be harmful if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label wherepossible) Risk Phrases: R22- Harmful if swallowed Hazard Phrases: H301 Precautionary Phrases: P301 + P310 |
| Cite This Product | Myriocin (ISP-1) (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-233) |
Biological Description
| Alternative Names | (2S,3R,4R,6E)-2-Amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxo-6-icosenoic acid |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 6438394 |
| Scientific Background | Myriocin, also known as ISP-1, is a potent inhibitor of serine palmitoyltransferase, the enzyme responsible for the first step in sphingolipid biosynthesis. In neuroscience, Myriocin is used to study the impact of sphingolipid metabolism on neuronal survival, inflammation, and synaptic function. Its immunosuppressive properties and ability to modulate lipid signaling pathways make it relevant in models of neuroinflammation and neurodegeneration. |
| References |
1. Hidari K.I.P.J., et al. (1996) J Biol Chem. 271: 14636-14641. 2. Miyake Y., Kozutsumi Y., Nakamura S., Fujita T. and Kawasaki T. (1995) Biochem Piophys Res Commun. 211(2): 396-403. |

Reviews
There are no reviews yet.