| Product Name | Nutlin-3 |
| Description |
p53/mdm2 inhibitor |
| Purity | >98% |
| CAS No. | 548472-68-0 |
| Molecular Formula | C30H30Cl2N4O4 |
| Molecular Weight | 581.9 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in 100 mM DMSO and Ethanol |
| Source | Synthetic |
| Appearance | Yellow solid |
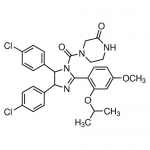
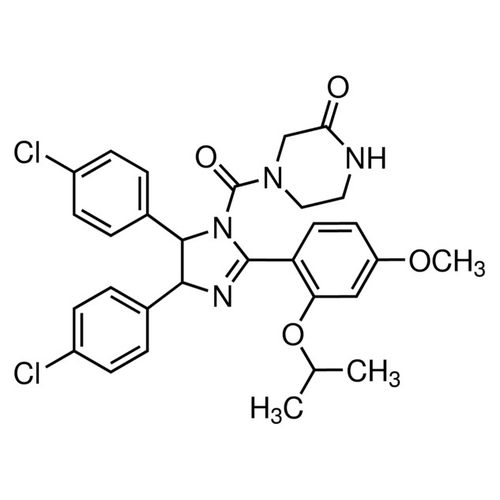
| SMILES | CC(C)OC1=C(C=CC(=C1)OC)C2=N[C@H]([C@H](N2C(=O)N3CCNC(=O)C3)C4=CC=C(C=C4)Cl)C5=CC=C(C=C5)Cl |
| InChI | InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37) |
| InChIKey | BDUHCSBCVGXTJM-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution – Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25- Avoid contact with skin and eyes |
| Cite This Product | Nutlin-3 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-344) |
Biological Description
| Alternative Names | (±)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5- dihydro-imidazole -1-carbonyl]-piperazin-2-one |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, p53/mdm2 Inhibitors |
| PubChem ID | 216345 |
| Scientific Background | Nutlin-3 is a potent and selective antagonist of MDM2 that inhibits the MDM2-p53 interaction (IC50 = 0.09 µM), leading to p53 stabilization and activation. It induces apoptosis and cell cycle arrest in cancer cells and has been widely used to study p53 signaling. In neuroscience, Nutlin-3 is of interest for its ability to modulate p53-dependent pathways involved in neuronal stress responses, DNA repair, and apoptosis. Its neuroprotective potential is being explored in models of stroke, neuroinflammation, and neurodegenerative diseases where p53 dysregulation contributes to pathology. |
| References | 1. Vassilev et al (2004) Science 303: 844. |

Reviews
There are no reviews yet.