| Product Name | Pridopidine |
| Description |
Neuroprotective |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 346688-38-8 |
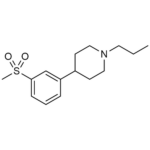
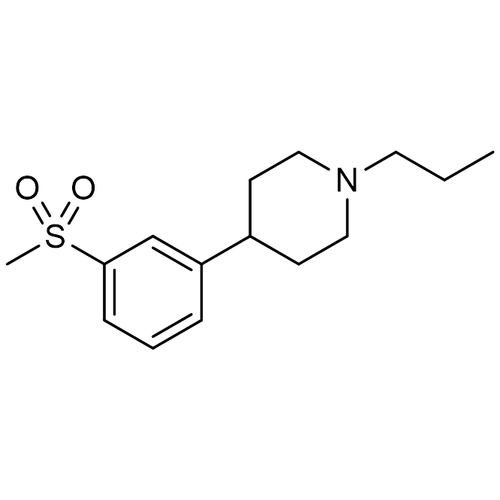
| Molecular Formula | C15H23NO2S |
| Molecular Weight | 281.4 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (10 mg/ml) |
| Source | Synthetic |
| Appearance | White powder |
| SMILES | CCCN1CCC(C2=CC(S(C)(=O)=O)=CC=C2)CC1 |
| InChI | 1S/C15H23NO2S/c1-3-9-16-10-7-13(8-11-16)14-5-4-6-15(12-14)19(2,17)18/h4-6,12-13H,3,7-11H2,1-2H3 |
| InChIKey | YGKUEOZJFIXDGI-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Warning GHS Hazard Statements,: H302, H361d Precautionary Statement Codes,: P203, P264, P270, P280, P301+P317, P318, P330, P405, and P501 |
| Cite This Product | Pridopidine (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-626) |
Biological Description
| Alternative Names | 4-(3-(Methylsulfonyl)phenyl)-1-propylpiperidine; ACR16 |
| Research Areas | ALS Disease, Neurodegeneration, Neuroscience |
| PubChem ID | 9795739 |
| Scientific Background | Pridopidine is a small molecule with neuroprotective potential, primarily investigated for Huntington’s disease, Parkinson’s disease, and schizophrenia. Initially described as a dopamine stabilizer due to its ability to modulate dopaminergic tone without inducing catalepsy, its mechanism is now attributed to high-affinity binding at the sigma-1 receptor. This receptor is implicated in cellular stress responses, neuroprotection, and synaptic plasticity. Pridopidine has demonstrated the ability to restore neuronal function, enhance neurotrophic signaling, and reduce neurodegeneration in preclinical models. Its sigma-1-mediated effects also extend to models of amyotrophic lateral sclerosis (ALS), highlighting its broad therapeutic potential in neurodegenerative disease research. |
| References |
1. F Pettersson et al. 2010 J. Med. Chem. 53:2510. 2. S Natesan et al. 2006 J. Pharmacol. Exp. Ther. 318:810. 3. T Dyhring et al. 2010 Eur. J. Pharmacol. 628:19. 4. K Sahlholm et al. 2015 Psychopharmacology (Berl) 232:3443. 5. K Sahlholm et al. 2013 Mol. Psychiatry 18:12. 6. V Francardo et al. 2019 Neurotherapeutics 16:465. 7. D Ryskamp et al. 2017 Neurobiol. Dis. 7 97(Pt A):46. 8. A Ionescu et al. 2019 Cell Death Dis. 10:210. |

Reviews
There are no reviews yet.