| Product Name | Santacruzamate A |
| Description |
Ultrapotent HDAC2 inhibitor |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 1477949-42-0 |
| Molecular Formula | C15H22N2O3 |
| Molecular Weight | 278.4 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (25 mg/ml); or Ethanol (20 mg/ml) |
| Source | Synthetic |
| Appearance | Off-white powder |
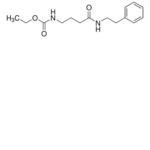
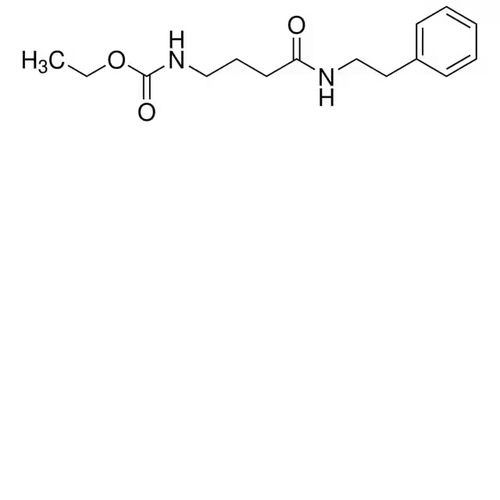
| SMILES | O=C(CCCNC(OCC)=O)NCCC1=CC=CC=C1, O=C(CCCNC(OCC)=O)NCCC1=CC=CC=C2, O=C(CCCNC(OCC)=O)NCCC1=CC=CC=C3 |
| InChI | InChI=1S/C15H22N2O3/c1-2-20-15(19)17-11-6-9-14(18)16-12-10-13-7-4-3-5-8-13/h3-5,7-8H,2,6,9-12H2,1H3,(H,16,18)(H,17,19) |
| InChIKey | HTOYBIILVCHURC-UHFFFAOYSA-N |
| Safety Phrases | GHS Hazard Statements: Not Classified |
| Cite This Product | Santacruzamate A (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-613) |
Biological Description
| Alternative Names | N-[4-Oxo-4-[(2-phenylethyl)amino]butyl]-carbamic acid, ethyl ester |
| Research Areas | Alzheimer's Disease, Apoptosis, Cancer, Neurodegeneration, Neuroscience |
| PubChem ID | 72946782 |
| Scientific Background | Santacruzamate A is a highly selective histone deacetylase 2 (HDAC2) inhibitor derived from marine cyanobacteria. It has been studied for its neuroprotective effects in Alzheimer’s disease models, where it attenuates amyloid-beta-induced toxicity and enhances endoplasmic reticulum stress tolerance. Its selectivity for HDAC2 over HDAC6 makes it a promising candidate for epigenetic modulation in neurodegenerative disease therapy, with potential applications in memory enhancement and synaptic plasticity. |
| References |
1. Pavlik C.M., et al. (2013) J. Nat. Prod. 76:2026 2.Zhou et al. H., (2018) Cell Prolif. 51(3):e12477 3. Damaskos C., et al. (2017) Anticancer Res. 37:35 4.Chen L., et al. (2019) Front. Cell. Neurosci. 13:61 |

Reviews
There are no reviews yet.