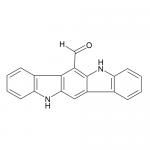
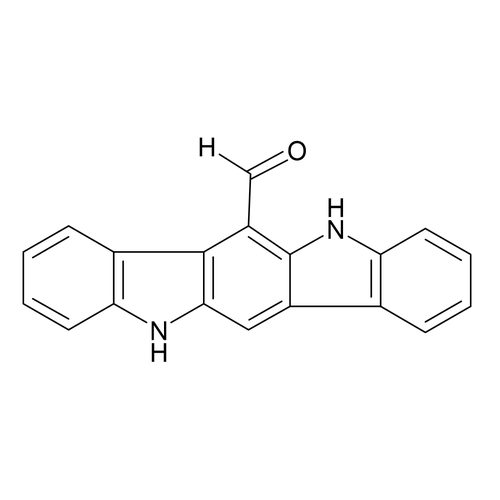
| Product Name | 6-Formylindolo[3,2-b]carbazole (FICZ) |
| Description |
Ah receptor ligand |
| Purity | >95% |
| CAS No. | 172922-91-7 |
| Molecular Formula | C19H12N2O |
| Molecular Weight | 284.3 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Ligand |
| Solubility | Soluble in DMSO (10 mg/ml) |
| Source | Synthetic |
| Appearance | Yellow solid |
| SMILES | C1=CC=C2C(=C1)C3=CC4=NC5=CC=CC=C5C4=C(C3=N2)C=O |
| InChI | InChI=1S/C19H10N2O/c22-10-14-18-12-6-2-4-8-16(12)20-17(18)9-13-11-5-1-3-7-15(11)21-19(13)14/h1-10H |
| InChIKey | OTWFDHQTFRXSAK-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution. Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25 - Avoid contact with skin and eyes |
| Cite This Product | 6-Formylindolo(3,2-b)carbazole (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-383) |
Biological Description
| Alternative Names | Indolo[3,2-b]carbazole-6-carbaldehyde, FICZ |
| Research Areas | Cell Signaling |
| PubChem ID | 22245026 |
| Scientific Background | 6-Formylindolo(3,2-b)carbazole (FICZ) is a high-affinity endogenous ligand for the aryl hydrocarbon receptor (AhR), derived from tryptophan metabolism. FICZ rapidly induces cytochrome P450 enzymes, particularly CYP1A1, at picomolar concentrations, highlighting its potency in modulating gene expression. In neuroscience, FICZ is gaining attention for its role in regulating neuroimmune interactions and oxidative stress via AhR signaling. These pathways are increasingly implicated in neurodegenerative diseases, including multiple sclerosis and Alzheimer’s disease. FICZ’s ability to influence neuroinflammation and cellular detoxification positions it as a promising molecule for studying environmental and metabolic contributions to brain health. |
| References |
1. Wei Y.D., et al. (1998) Chem. Biol. Interact. 110(1-2): 39-55. 2. Rannug U., et al. (1995) Chem. Biol. Drug. Des. 2(12): 841-855. 3. Rannug A., et al. (1987) J. Biol. Chem. 262(32): 15422-7. |

Reviews
There are no reviews yet.