| Product Name | Zafirlukast |
| Description |
Hsp70 inhibitor |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 107753-78-6 |
| Molecular Formula | C31H33N3O6S |
| Molecular Weight | 575.7 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in 25 mg/ml DMSO |
| Source | Synthetic |
| Appearance | White to Pink Solid |
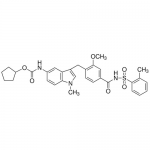
| SMILES | CC1=CC=CC=C1S(=O)(=O)NC(=O)C2=CC(=C(C=C2)CC3=CN(C4=C3C=C(C=C4)NC(=O)OC5CCCC5)C)OC |
| InChI | InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32 |
| InChIKey | YEEZWCHGZNKEEK-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution- Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25- Avoid contact with skin and eyes |
| Cite This Product | Zafirlukast (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-361) |
Biological Description
| Alternative Names | Cyclopentyl [3-(2-methoxy-4-{[(2-methylphenyl)sulfonyl]carbamoyl}benzyl)-1-methyl-1H-indol-5-yl]carbamate; ICI-204219 |
| Research Areas | Cancer, Heat Shock |
| PubChem ID | 5717148 |
| Scientific Background |
Zafirlukast, a leukotriene receptor antagonist and HSP70 inhibitor (IC50 = 37 µM), is primarily known for asthma treatment but is gaining interest in neurodegenerative disease research. HSP70 is a molecular chaperone essential for protein homeostasis and stress response—processes disrupted in Alzheimer’s, Parkinson’s, and Huntington’s diseases. Zafirlukast’s ability to modulate HSP70 activity and cross the blood-brain barrier makes it a promising candidate for targeting proteostasis and neuroinflammation. Its dual role in immunology and neurobiology underscores its potential for repurposing in neuroprotective strategies. |
| References | 1. Miyata Y., et al. (2010) J. Biomol. 15: 1211. |

Reviews
There are no reviews yet.