Properties
| Storage Buffer | 20mM HEPES buffer pH7.2, 80mM NaCl, 10% glycerol |
| Storage Temperature | -20ºC |
| Shipping Temperature | Blue Ice or 4ºC |
| Purification | Affinity Purified |
| Cite This Product | Human Recombinant p23 Protein (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-303) |
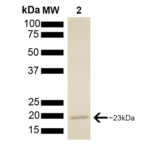
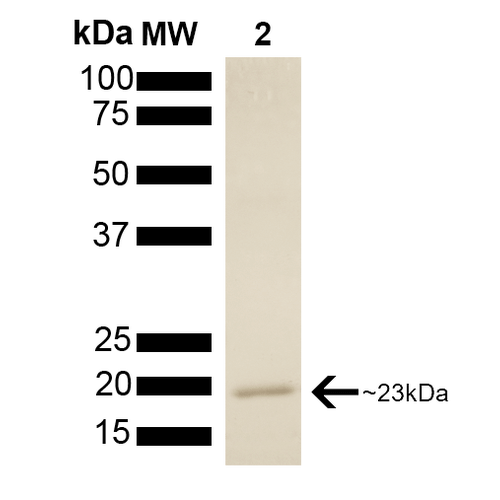
| Certificate of Analysis | This product has been certified >90% pure using SDS PAGE analysis. 4uM SPR-303, when added to 2uM SPR-300 (Aha1)-activated HSP90 (2uM; His-tagged HSP90 beta) in 33mM Hepes pH7.2, 30mM NaCl, 5mM MgCl2, 1mM DTT, 1.5mM ATP in a 100ul reaction at 37 degrees C, eliminated all Aha1-mediated ATPase stimulation as well as intrinsic HSP90 ATPase activity. (This is an enzyme-linked ATP regeneration assay tracking loss of NADH absorbance at 340nm). |
Biological Description
| Alternative Names | Sid 3177, Co chaperone p23, cPGES, HSP90 co chaperone, cytosolic prostaglandin E2 synthase, PTGES3, TEBP, Prostaglandin E synthase 3 |
| Research Areas | Cancer, Heat Shock |
| Cellular Localization | Cytoplasm |
| Accession Number | NP_006592.3 |
| Gene ID | 10728 |
| Swiss Prot | Q15185 |
| Scientific Background |
p23 is a small co-chaperone that stabilizes the ATP-bound conformation of HSP90, promoting the maturation of client proteins and preventing premature release. It plays a key role in the late stages of the HSP90 chaperone cycle, ensuring proper folding and functional activation of a wide range of signaling proteins. In neuroscience, p23 is involved in the regulation of neuronal development, synaptic plasticity, and stress responses. Its interaction with HSP90 is critical for maintaining the stability of proteins implicated in neurodegenerative diseases, including tau, glucocorticoid receptors, and kinases. Reduced p23 expression has been associated with increased protein aggregation and cellular stress in models of Alzheimer’s disease. Conversely, enhancing p23 function may improve proteostasis and reduce neurotoxicity. Its role in modulating the chaperone machinery positions p23 as a potential biomarker and therapeutic target in neurodegeneration. |
| References |
1. Johnson J.L., Beito T. G., Krco C.J. & Toft D.O. (1994) Mol Cell Biol. 14: 1956-63. 2. Weikl T., Abelmann K. & Buchner J. (1999) J Mol Biol. 293: 685-91. 3. Weaver A.J., Sullivan W.P., Felts S.J., Owen B.A. & Toft, D.O. (2000) J Biol Chem. 275: 23045-52. 4. Nair S.C., et al. (1996) Cell Stress Chaperones. 1: 237-50. 5. Xu Z., et al. (1997) Eur J Biochem. 246, 461-70. 6. Holt S.E., et al. (1999) Genes Dev. 13: 817-26. 7. Hu J., Toft D., Anselmo D. & Wang X. (2002) J Virol. 76: 269-79. 8. Felts S.J. & Toft D.O. (2003) Cell Stress Chaperones. 8: 108-13. 9. Gausdal G., Gjertsen B.T., Fladmark K.E., Demol H., Vandekerckhove J. & Doskeland S.O. (2004) Leukemia. |





















StressMarq Biosciences :
Based on validation through cited publications.