Alpha Synuclein Inhibition Using Biomimetic Peptides
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, characterized by the gradual loss of neurons in the substantia nigra pars compacta—a region of the brain responsible for controlling movement. As these neurons degenerate, their ability to produce a key neurotransmitter, dopamine, declines. This biochemical imbalance gives rise to the hallmark clinical symptoms of Parkinson’s disease, including resting tremors, muscle rigidity, and bradykinesia (slowness of movement).
A defining pathological feature of Parkinson’s disease is the accumulation of alpha synuclein. Under disease conditions, the normally monomeric, physiological form of alpha synuclein aggregates into toxic fibrils, which subsequently form Lewy bodies (LBs). These aggregates not only induce neuronal cell death but also propagate to neighboring cells, exacerbating neurodegeneration.
In addition, the progression of PD has been closely linked to oxidative stress, which results from cellular damage caused by free radicals. Oxidative modifications, such as nitrosylation and tyrosine dimerization, are capable of accelerating alpha synuclein aggregation. Furthermore, oxidative stress may indirectly promote aggregation by impairing protein degradation pathways, including proteasome and autophagy systems.
To counter these effects, researchers are exploring dual-functional peptides that combine antioxidant properties with the ability to inhibit fibril formation. These peptides can be chemically conjugated to specifically target alpha synuclein aggregates, offering a promising therapeutic strategy to address the complex cellular signaling impairments associated with Parkinson’s disease.
Biomimetic peptides as therapeutics
Biomimetic peptides are synthetic peptides designed to mimic the structure and function of naturally occurring peptides. A well-known example is exendin-4, originally derived from the saliva of the Gila monster lizard (Heloderma suspectum), which functions as a glucagon-like peptide-1 (GLP-1) receptor agonist. Its synthetic counterpart, exenatide, has been developed into a commercial drug and is widely used in the treatment of type 2 diabetes.
In a study published in Biomimetics by Frantzeskos et al., researchers from Fordham University in New York designed biomimetic peptides targeting Parkinson’s disease pathology. These peptides were shown to induce conformational changes in pathogenic alpha synuclein pre-formed fibrils (PFFs), resulting in a significant reduction in their fibrillar structure over time.
Importantly, the biomimetic peptides demonstrated dual functionality. In addition to blocking alpha synuclein fibril formation, they exhibited promising antioxidant properties. While these findings were encouraging, further research was needed to evaluate their effects in cellular models and as neuroprotective, disease-modifying agents, either as standalone treatments or in combination with other therapeutic compounds.
Computational peptide modelling
To initiate the study, researchers investigated marine-derived natural peptides, which are well known for their antioxidant properties. The initial focus was on bioactive peptides sourced from mussels and ark clam shells. One peptide of particular interest was PIIVYWK, derived from the Blue mussel (Mytilus edulis), which has been demonstrated to possess antioxidant properties that assist in the mitigation of oxidative stress and inflammation.
To enhance its functionality, researchers introduced targeted mutations within the peptide sequence. Key modifications included replacing two isoleucine residues with tyrosine and removing valine, resulting in improved antioxidant potential. Additionally, fibril inhibitory motifs (FIMs), specifically, ELAQM and DPNGS, were appended to the N-terminus, C-terminus, or both ends of the peptide to further enhance its anti-aggregation properties.
Subsequently, a series of molecular docking and molecular dynamics simulations was conducted in silico to evaluate the interactions between the modified peptides and both pre-fibrillar and fibrillar forms of alpha synuclein. The peptides were found to interact with residues in the non-amyloid-β component (NAC) domain of alpha synuclein, a region critical for fibril formation.
To ensure that the peptides possessed a low propensity for self-aggregation, predictive tools such as AGGRESCAN, a server for the predication of protein aggregation propensity, were used to identify aggregation-prone regions. This computational screening helped confirm the peptides’ suitability for further development as dual-functional agents with both antioxidant and fibril-inhibiting capabilities.
Harnessing StressMarq’s neurodegenerative proteins to advance peptide-based therapies
In order to further validate the potential of a selected subset of engineered peptides, researchers turned their focus to laboratory-based trials. Five peptides were chosen based on their performance in computational modeling studies. Data from surface plasmon resonance (SPR) confirmed the modeling predictions, demonstrating that these peptides effectively bound to alpha synuclein fibrils.
To assess their ability to inhibit fibril formation, researchers introduced Thioflavin T (ThT), a dye that binds to beta-sheet-rich fibrillar structures. In tandem with StressMarq’s Alpha Synuclein Pre-formed Fibrils (Type 1) (catalog #SPR-322), the assay revealed that peptide treatment led to a time-dependent decrease in fluorescence, indicating a reduction in fibrillar alpha synuclein and successful inhibition of fibril formation compared to untreated controls.
Finally, for these peptides to be viable as neurological therapeutics, it was important that they were able to effectively cross the blood-brain barrier (BBB). This presents a major challenge for many drug candidates, particularly for those of a larger size. Using ADMETlab computational software, researchers evaluated the BBB permeability of the peptides. The results were favorable, with most peptides predicted to efficiently cross the BBB, further highlighting their potential as therapeutic agents for neurodegenerative diseases.
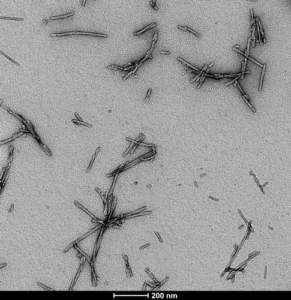
[Image from: StressMarq website] Transmission electron microscopy (TEM) imaging of Alpha Synuclein PFFs (Type 1) (catalog# SPR-322).
Summary
By adopting three-dimensional structures, peptide-based drugs represent a promising new class of therapeutics that can be precisely tailored to target specific biological sites and exert disease-modifying effects, enhancing therapeutic efficacy while minimizing off-target interactions. Unlike antibodies, which often struggle to cross the blood-brain barrier and are costly to produce, peptide-based therapies are generally more permeable and less expensive to manufacture.
The dual-functional peptides described in this study offer a compelling alternative for treating Parkinson’s disease, a condition characterized by multiple pathological mechanisms, including protein aggregation and oxidative stress. This research highlights the design of biomimetic peptides that combine antioxidant activity with the ability to inhibit the formation of pathogenic alpha synuclein fibrils.
In silico computational modelling, alongside in vitro fibrillization assays confirmed that these peptides effectively reduced the aggregation of StressMarq’s Alpha Synuclein Pre-formed Fibrils (Type 1) (catalog #SPR-322). The published study by Frantzeskos et al. successfully developed peptides with dual functionality, offering a promising strategy to target the complex molecular pathways that are impaired in neurodegenerative diseases.
Related StressMarq Products
StressMarq manufactures a diverse array of neurodegenerative proteins to support preclinal drug discovery research for the treatment of neurodegenerative diseases. Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau and amyloid beta pre-formed fibrils, oligomers, and monomers for neurodegenerative disease research.
References
- Exploring the potential of biomimetic peptides in targeting fibrillar and filamentous alpha-synuclein—An in silico and experimental approach to Parkinson’s disease. Frantzeskos, S. A. et al., Biomimetics. 2024.

Leave a Reply