Alpha Synuclein SUMOylation: A Novel Therapeutic Target for Parkinson’s Disease
StressMarq’s mouse alpha synuclein pre-formed fibrils (PFFs) (catalog# SPR-324) were recently cited in a study published in eNeuro journal. A research group from Delaware State University used our mouse alpha synuclein (Type 1) PFFs to induce toxicity in N27 rat dopaminergic cells and investigate the effects of SUMOylation on alpha synuclein aggregation and toxicity. The findings of this research demonstrated that regulation of SUMO conjugation to alpha synuclein could be a target to protect dopaminergic neurons from oxidative stress and protein aggregation, both of which are implicated in Parkinson’s disease progression.
What is SUMOylation?
SUMOylation is a reversible post-translational modification similar to ubiquitination. In contrast to ubiquitin, which mainly tags proteins for degradation, SUMOylation is essential for normal cellular processes including cell cycle regulation, nuclear-cytosolic transport, gene transcription, protein stability, response to stress, apoptosis and many others. SUMO (small ubiquitin-like modifier) is a small protein (~12kDa), covalently attached to lysine residues of target proteins in order to modify their function. SUMO may also compete with ubiquitin for the same lysine residue which results in protecting the protein from degradation1. SUMOylation can affect the protein structure, stability, localization and protein-protein interaction2. This post translational modification has been shown to target several proteins related to neurodegeneration such as alpha synuclein and tau3.
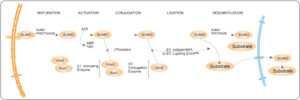
The SUMO conjugation pathway
The SUMO conjugation pathway involves 3 enzymes: SUMO activating enzyme (SAE1), Ubc9 conjugating enzyme and SUMO-E3 ligase4. First, SUMO binds to the E1 activating enzyme complex (SAE1 and SAE2) which activates the reaction process. The activated SUMO is transferred to the E2 conjugating enzyme or Ubc9 which binds SUMO via its cysteine residue. The E3 ligase catalyzes the conjugation of SUMO to a substrate. SUMO can be recycled for conjugation to other proteins multiple times5.
How does SUMOylation affect alpha synuclein?
Alpha synuclein is the major component of Lewy bodies in Parkinson’s disease which is characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) leading to motor dysfunction. Alpha synuclein undergoes several post-translational modifications including phosphorylation, ubiquitination, nitration, acetylation, truncation and SUMOylation which all play a role in protein’s aggregation and toxicity. Specifically, Ubc9 conjugase covalently attaches a SUMO protein to alpha synuclein at lysine residues. Several studies have demonstrated the effects of SUMOylation on alpha synuclein. SUMO, which is highly soluble, has been shown to enhance the solubility of aggregation-prone proteins like alpha synuclein and impaired SUMOylation increased alpha synuclein aggregation and toxicity in HEK293 cells and a PD rat model6. Another study showed that Ubc9 overexpression increased the solubility of alpha synuclein and prevented methamphetamine-induced protein aggregation. Furthermore, non-SUMO mutants of alpha synuclein enhanced their aggregation by impairing degradation through the UPS (ubiquitin-proteasome system) and ALP (autophagy–lysosomal pathway) in vitro and in vivo7.
Alpha synuclein SUMOylation as a therapeutic target for Parkinson’s disease
The new study by Verma et al. showed that Ubc9-mediated SUMOylation is neuroprotective against the neurotoxin MPTP (MPP+, 1-methyl-4-phenylpyridinium) or alpha synuclein preformed fibril-induced toxicities in vitro and in vivo. Specifically, the researchers used N27 rat dopaminergic cell lines overexpressing Ubc9-EGFP or EGFP and treated them with different concentrations of the neurotoxin MPTP. They found that Ubc9 overexpression protected the cells from the neurotoxin and reduced ROS production. The cells were also exposed to StressMarq mouse alpha synuclein PFFs (catalog# SPR-324) to assess the effects of Ubc9 overexpression or knock-down (Ubc9-RNAi) against PFF- induced toxicity. Adding alpha synuclein PFFs to cells can trigger endogenous alpha synuclein aggregation and seed Lewy body pathology. The results from the cytotoxicity (LDH) and cell viability (MTT) assays demonstrated that Ubc9 overexpression significantly protected the dopaminergic cells from PFF toxicity and ROS generation. On the other hand, Thioflavin T staining showed that RNAi-mediated Ubc9 knock-down significantly enhanced PFF-induced alpha synuclein aggregation. Moreover, Ubc9 overexpression in N27 cells increased the endogenous level of α-synuclein almost 49% suggesting that SUMOylated alpha synuclein avoids normal protein degradation.
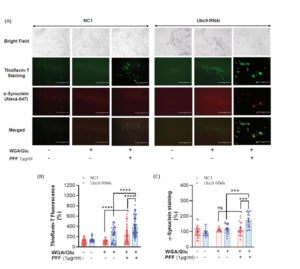
Ubc9 knock-down by RNAi exacerbates PFF-induced protein aggregation in Thioflavin T staining. A. Bright field images show the location of N27 cells (top row). Immunocytochemical images of Thioflavin T staining in dark field show PFF-induced protein aggregation in green fluorescent label (2nd row). Alpha-synuclein staining (red) in immunocytochemistry was detected in Thioflavin T labeled protein aggregates (3rd row). The merged images (yellow, the bottom row) demonstrate that α-synuclein is co-localized with Thioflavin T stained protein aggregates. B. PFF exposure to N27 cells for 24 h results in the accumulation of protein aggregation labeled in Thioflavin T. Ubc9-RNAi further enhances PFF- induced protein aggregation, compared to NC1 random cocktail control with WGA/GluNAc. C. PFF increases the level of α-synuclein in Thioflavin T-positive aggregates with Ubc9-RNAi, and Ubc9 knock down aggravates α-synuclein accumulation in the protein aggregates compared to NC1 control after PFF treatment, although PFF did not significantly increase the level of α-synuclein in the aggregates in NC1 control treated cells.
The researchers also tested MPTP toxicity in Ubc9 transgenic mice which were injected with the neurotoxin once a day for seven days. Ubc9 transgenic mice showed significant protection of the dopaminergic neurons in the striatum and SNc from MPTP induced toxicity compared to the wild type.
SUMOylation has recently gained more attention in drug discovery as many studies revealed its neuroprotective role in several neurodegenerative diseases. The results of this recent study clearly show that regulating SUMOylation of alpha synuclein can be a novel therapeutic target to prevent Lewy bodies formation and ROS generation in Parkinson’s disease.
REFERENCES
- SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Gill G. (2004) Genes Dev.18(17):2046–59.
- SUMOylation in α-Synuclein Homeostasis and Pathology. Savyon M. et al. (2020) Front. Aging Neurosci. 12:167.
- Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. Dorval V., and Fraser P.E. (2006). J. Biol. Chem. 281: 9919–9924.
- Mechanisms, regulation and consequences of protein SUMOylation. Wilkinson K.A. et al. (2010) Biochemical journal 428(2):133-45.
- SUMO-regulated mitochondrial function in Parkinson’s disease. de Souza A. et al. (2016) Journal of Neurochemistry. 137: 673-686.
- Sumoylation inhibits α-synuclein aggregation and toxicity. Krumova P. et al. (2011) J Cell Biol 194(1): 49-60.
- SUMOylation of Alpha-Synuclein Influences on Alpha-Synuclein Aggregation Induced by Methamphetamine. Zhu L. et al. (2018) Front. Cell. Neurosci. 12:262.


Leave a Reply